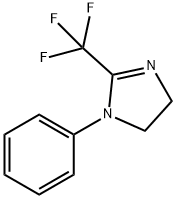
1-Phenyl-2-(trifluoroMethyl)-4,5-dihydro-1H-iMidazole synthesis
- Product Name:1-Phenyl-2-(trifluoroMethyl)-4,5-dihydro-1H-iMidazole
- CAS Number:1069085-41-1
- Molecular formula:C10H9F3N2
- Molecular Weight:214.19
Yield:1069085-41-1 82%
Reaction Conditions:
with tetrachloromethane;triethylamine;triphenylphosphine at 0; for 18 h;Reflux;Inert atmosphere;
Steps:
2. General Procedure for the Synthesis of N-substituted-2-fluoroalkyl substituted imidazolines (2, 3)
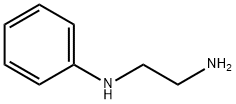
General procedure: A 200-mL three-necked flask equipped with a condenser was charged with Ph3P (34.5 g, 132mmol), Et3N (excess), CCl4 (21.1 ml, 220 mmol), and the fluorinated carboxylic acid (44 mmol) at 0 °C under nitrogen. After the solution was stirred for about 10 min (ice water bath), thenN-substituted alkyl diamine or 2-amino-3-phenyl propanol (53 mmol) dissolved in CCl4 (21.1 ml, 220 mmol) was added. The mixture was refluxed under stirring for 3-18 h. Solvent was evaporatedunder reduced pressure, and the residue was diluted with petroleum ether (60-90 °C) and filtered.The residual solid of Ph3PO and Et3N*HCl was washed with petroleum ether 3 times. The filtratewas concentrated, and the residue was then purified by column chromatography to offer the product 3. When N-benzylethylenediamine and N-phenylethylenediamine were employed, imidoyl chloride2b, 2f, 2g, 2m can be isolated by shorting the stirring time to 1-2 h.
References:
Jiang, Haizhen;Sun, Lan;Yuan, Shijie;Lu, Wenjun;Wan, Wen;Zhu, Shizheng;Hao, Jian [Tetrahedron,2012,vol. 68,# 13,p. 2858 - 2863] Location in patent:supporting information; experimental part