
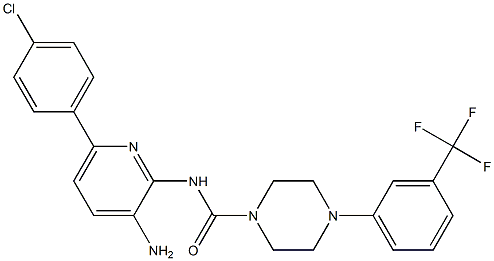
(5-(4-chlorophenyl)-3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)(4-(3-(trifluoroMethyl)phenyl)piperazin-1-yl)Methanone synthesis
- Product Name:(5-(4-chlorophenyl)-3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)(4-(3-(trifluoroMethyl)phenyl)piperazin-1-yl)Methanone
- CAS Number:1072874-79-3
- Molecular formula:C23H18ClF3N6O
- Molecular Weight:486.88
1072874-82-8
0 suppliers
inquiry
![(5-(4-chlorophenyl)-3H-[1,2,3]triazolo[4,5-b]pyridin-3-yl)(4-(3-(trifluoroMethyl)phenyl)piperazin-1-yl)Methanone](/CAS/GIF/1072874-79-3.gif)
1072874-79-3
23 suppliers
$223.00/25mg
Yield:1072874-79-3 26%
Reaction Conditions:
with hydrogenchloride;sodium nitrite in water at 0;Cooling;
Steps:
1.4
1.4. [5-(4-chlorophenyl)[1,2,3]triazolo[4,5-b]pyridin-3-yl][4-(3-trifluoromethylphenyl)piperazin-1-yl]methanone In a 100 ml three-necked flask, 0.55 g (1.17 mmol) of 4-(3-trifluoromethylphenyl)piperazine-1-carboxylic acid, [3-amino-6-(4-chlorophenyl)pyridin-2-yl]amide, obtained in stage 1.3, is dissolved in a mixture of 5 ml of water and 5 ml of 37% aqueous hydrochloric acid, cooled in an ice bath. Using a dropping funnel, 0.24 g (3.50 mmol) of sodium nitrite dissolved in 4 ml of water is added dropwise. The mixture is stirred for 2 hours at 0° C. It is left to stand for one hour in the ice bath and then the solid is filtered off. It is washed with 3*10 ml of water so as to obtain a beige solid. The latter is dried under vacuum in the presence of phosphorus pentoxide. The product is purified by silica gel chromatography, elution being carried out with an 80:20 then 60:40 mixture of cyclohexane and ethyl acetate. The oil obtained is crystallized from pentane so as to obtain 0.15 g (yield: 26%) of product in the form of a light yellow powder. Melting point (° C.): 139-141° C. LC-MS (m/z): 487 (MH+) IR (KBr, cm-1): 1718, 1591 1H NMR (DMSO-d6, δ ppm): 8.80 (d, 1H), 8.25 (dd, 3H), 7.60 (d, 2H), 7.45 (t, 1H), 7.25 (d, 1H), 7.20 (s, 1H), 7.10 (d, 1H), 3.30-4.00 (m, 8H).
References:
US2010/41670,2010,A1 Location in patent:Page/Page column 4