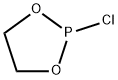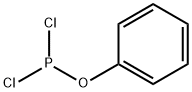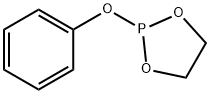
Phosphorous acid, cyclic ethylene ester, phenyl ester synthesis
- Product Name:Phosphorous acid, cyclic ethylene ester, phenyl ester
- CAS Number:1077-05-0
- Molecular formula:C8H9O3P
- Molecular Weight:184.13
Yield:1077-05-0 94%
Reaction Conditions:
Stage #1: phenol in dichloromethane at 20; for 0.5 h;
Stage #2: 2-chloro-1,3,2-dioxaphospholan in dichloromethane at 0 - 20; for 13.5 h;
Steps:
1 Example 1Preparation of Compound 1 (Phenyl Phosphite Phosphite or 2-Phenyl-1,3,2-Dioxolane):
Add to 500mL three-necked flask at room temperature200 g of dichloromethane (solvent,Same effect as the aforementioned non-aqueous solvent,(Optionally) and 100 g of phenol (reactant C),Stir at room temperature for 0.5h to completely dissolve the phenol.The solution is colorless and transparent,The three-necked bottle is connected to an external gas absorption device,Subsequently, 127 g of reaction intermediate B was slowly added to a 500 mL three-neck via a dropping funnel.Control the drop acceleration to 1 drop / second,The reaction temperature is 0 ° C.With the dropwise addition of reaction intermediate B,Emit a lot of heat and a lot of HCl gas,The solution was colorless and transparent after all the dripping (droppingReaction intermediate B so far 1.5h),Then warmed to room temperature and continued to stir for 12 hours.The dichloromethane was then removed to give a pale yellow liquid,The light yellow liquid was distilled under reduced pressure at 150 ° C to obtain 173 g of a colorless transparent liquid with a yield of 94%.This product is Compound 1(Phenyl vinyl phosphite or 2-phenolyl-1,3,2-dioxocyclopentane).
References:
CN110563764,2019,A Location in patent:Paragraph 0063; 0064; 0065
7114-39-8
0 suppliers
inquiry

108-95-2
727 suppliers
$14.00/25g

1077-05-0
3 suppliers
inquiry

124-40-3
504 suppliers
$18.00/100ml