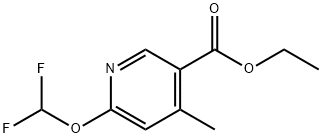
Ethyl Ester 6-(Difluoromethoxy)-4-methyl-3-pyridinecarboxylic Acid synthesis
- Product Name:Ethyl Ester 6-(Difluoromethoxy)-4-methyl-3-pyridinecarboxylic Acid
- CAS Number:1079352-16-1
- Molecular formula:C10H11F2NO3
- Molecular Weight:231.2

64-17-5
708 suppliers
$10.00/50g
201230-82-2
1 suppliers
inquiry

1079352-13-8
20 suppliers
inquiry

1079352-16-1
2 suppliers
inquiry
Yield:-
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;1,1'-bis(diphenylphosphino)ferrocene;palladium diacetate in dimethyl sulfoxide at 90;Inert atmosphere;
Steps:
11
A mixed solution of dimethyl sulfoxide (11 mL)/ethanol (7.7 mL) of the compound prepared in Example10 (835 mg) and diisopropylethylamine (1.53 mL) was deaerated, and it was substituted in argon. After having added bis(diphenylphosphino)ferrocene (195 mg) and palladium acetate (79 mg) to the reaction mixture, the reaction mixture was deaerated. The reaction mixture was substituted in carbon monoxide, and stirred at 90°C overnight. The reaction mixture was subjected to filtration with Celite (trade name) after having been cooled to room temperature. The obtained filtrate was extracted with ethyl acetate. The organic layer was washed by a saturated aqueous solution of sodium chloride, and dried with magnesium sulfate. The organic layer was concentrated under reduced pressure. The obtained residue was purified by column chromatography on silica gel (high flash-SI, size L (made in Yamazen corporation)) to obtain a title compound (618 mg) having the following physical data. TLC:Rf 0.52 (ethyl acetate/n-hexane=1/5); 1H-NMR(CDCl3): δ 1.40 (t, J =7.2 Hz, 3 H), 2.63 (s, 3 H), 4.38 (q, J =7.2 Hz, 2 H), 6.76 (s, 1 H), 7.24-7.78 (m, 1 H), 8.74 (s, 1 H).
References:
EP2154139,2010,A1 Location in patent:Page/Page column 38