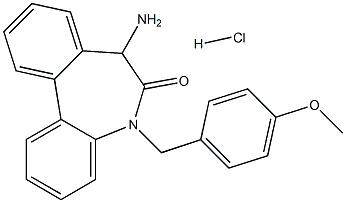
6H-Dibenz[b,d]azepin-6-one, 7-amino-5,7-dihydro-5-[(4-methoxyphenyl)methyl]-, hydrochloride (1:1) synthesis
- Product Name:6H-Dibenz[b,d]azepin-6-one, 7-amino-5,7-dihydro-5-[(4-methoxyphenyl)methyl]-, hydrochloride (1:1)
- CAS Number:1083065-04-6
- Molecular formula:C22H21ClN2O2
- Molecular Weight:380.8673
![5-(4-methoxybenzyl)-5H-dibenzo[b,d]azepine-6,7-dione-7-oxime](/CAS/20180702/GIF/847926-87-8.gif)
847926-87-8
1 suppliers
inquiry
![6H-Dibenz[b,d]azepin-6-one, 7-amino-5,7-dihydro-5-[(4-methoxyphenyl)methyl]-, hydrochloride (1:1)](/CAS/20180808/GIF/1083065-04-6.gif)
1083065-04-6
15 suppliers
inquiry
Yield:1083065-04-6 96%
Reaction Conditions:
with hydrogenchloride;palladium 10% on activated carbon;hydrogen in methanol;water at 50; under 7500.75 Torr; for 48 h;
Steps:
1.e
Aqueous HCl 2N (20.8 mL) was added to a solution of 5-(4-methoxy-benzyl)-5H-dibenzo[b,d]azepine-6,7-dione 7-oxime (8.3 g) in methanol (83.0 mL). Pd/C (10% weight, 540 mg) was added to the reaction mixture and the system was stirred under 10 bar H2 at 50° C. for 2 days. After exchanging H2 for Ar, the reaction mixture was filtered, concentrated under reduced pressure and dried under high vacuum yielding 7-amino-5-(4-methoxy-benzyl)-5H,7H-dibenzo[b,d]azepin-6-one hydrochloride (8.7 g, quant.) as a light yellow powder. This powder was suspended in acetonitrile (43 mL) and stirred 2 hours at room temperature. The precipitate was filtered off, washed with acetonitrile and dried under reduced pressure yielding 7-amino-5-(4-methoxy-benzyl)-5H,7H-dibenzo[b,d]azepin-6-one hydrochloride (8.3 g, 96%) as an off-white powder.MS(ISP): m/e=367 (M+Na+, 13), 345 (M+H+, 100)
References:
US2008/293933,2008,A1 Location in patent:Page/Page column 5-6