
2-Methoxy-6-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine synthesis
- Product Name:2-Methoxy-6-methyl-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)pyridine
- CAS Number:1083168-87-9
- Molecular formula:C13H20BNO3
- Molecular Weight:249.11
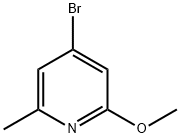
1083169-00-9
29 suppliers
inquiry

73183-34-3
581 suppliers
$6.00/5g

1083168-87-9
31 suppliers
$165.00/50mg
Yield:-
Reaction Conditions:
with potassium acetate;(1,1'-bis(diphenylphosphino)ferrocene)palladium(II) dichloride in N,N-dimethyl-formamide at 80; for 18 h;
Steps:
AA
AA. 2-Methoxy-6-methyl-4-(4.4.5.5-tetramethyl-1.3.2-dioxaborolan-2- vDpyridine; To 4-bromo-2-methoxy-6-methylpyridine (0.681 g, 3.37 mmoL), bis(pinacol)diboron (1.11 g, 4.38 mmoL), KOAc (0.992 g, 10.11 mmoL) and anhydrous DMF (21 mL), Pd(dppf)Cl2 (0.120 g, 0.163 mmoL) was added and stirred at 80 0C for 18 hours. The solvent was evaporated under reduced pressure. To the crude product, ethyl acetate (40 mL) and water (40 mL) were added. The biphasic mixture was filtered through a plug of celite and the layers were separated. The organic layer was dried over Na2SO4, filtered and evaporated under reduced pressure. The crude product was purified by column chromatography on silica gel (0-50% ethyl acetate in hexane) to yield 2-methoxy-6-methyl-4-(4,4,5,5-tetramethyl-l,3,2- dioxaborolan-2-yl)pyridine. ESI-MS m/z calc. 249.11, found 250.3 (M+l)+. Retention time 0.19 minutes.
References:
WO2008/141119,2008,A2 Location in patent:Page/Page column 99
63071-03-4
190 suppliers
$10.00/1g

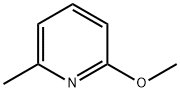
73183-34-3
581 suppliers
$6.00/5g

1083168-87-9
31 suppliers
$165.00/50mg