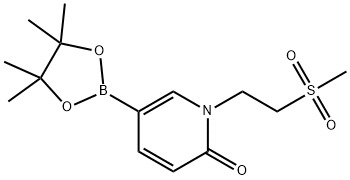
1-[2-(Methylsulfonyl)ethyl]-6-oxo-1,6-dihydropyridine-3-boronic Acid Pinacol Ester synthesis
- Product Name:1-[2-(Methylsulfonyl)ethyl]-6-oxo-1,6-dihydropyridine-3-boronic Acid Pinacol Ester
- CAS Number:1083168-89-1
- Molecular formula:C14H22BNO5S
- Molecular Weight:327.2
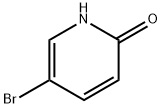
![5-Bromo-1-[2-(methylsulfonyl)ethyl]pyridin-2(1H)-one](/CAS/20180601/GIF/1083168-88-0.gif)
1083168-88-0
18 suppliers
$45.00/50mg

73183-34-3
578 suppliers
$6.00/5g
![1-[2-(Methylsulfonyl)ethyl]-6-oxo-1,6-dihydropyridine-3-boronic Acid Pinacol Ester](/CAS/20150408/GIF/1083168-89-1.gif)
1083168-89-1
28 suppliers
inquiry
Yield:1083168-89-1 90%
Reaction Conditions:
with potassium acetate;dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2 in N,N-dimethyl-formamide at 80; for 2 h;
Steps:
AD.b
Step b: l-(2-(Methylsulfonyl)ethyl)-5-(4, 4, 5, 5-tetramethyl-l, 3, 2-dioxaborolan-2- yl)pyridin-2 (IH) -one; 5-Bromo-l-(2-(methylsulfonyl)ethyl)pyridin-2(lH)-one (1.7 g, 6.0 mmol), potassium acetate (1.8 g, 18.1 mmol), bis(pinacolato)diboron (2.0 g, 7.8 mmol), and dichloro[l,l '-bis(diphenylphosphino)ferrocene]palladium (II) dichloromethane adduct (Pd(dppf)Cl2, 221.1 mg, 0.30 mmol) were combined in N,N-dimethylformamide (37 mL). The resulting reaction mixture was stirred and heated to 80 0C for 2 hours. The crude reaction mixture was evaporated to dryness, partitioned between 250 mL of ethyl acetate and 250 mL of water, filtered through celite, and the layers were separated. The organic layer was dried over sodium sulfate and then evaporated to dryness. The crude material was purified on silica gel (120 g) utilizing a gradient of 0-10% methanol in dichloromethane to yield the product as a semi-pure brown oil (1.78 g, 5.43 mmol, 90%). ESI-MS m/z calc. 327.1, found 328.2 (M+l)+. Retention time 0.90 minutes.
References:
WO2008/141119,2008,A2 Location in patent:Page/Page column 101
13466-38-1
390 suppliers
$6.00/5g
![1-[2-(Methylsulfonyl)ethyl]-6-oxo-1,6-dihydropyridine-3-boronic Acid Pinacol Ester](/CAS/20150408/GIF/1083168-89-1.gif)
1083168-89-1
28 suppliers
inquiry