
Boc-NH-PEG2-CH2COOH synthesis
- Product Name:Boc-NH-PEG2-CH2COOH
- CAS Number:108466-89-3
- Molecular formula:C11H21NO6
- Molecular Weight:263.29

24424-99-5
821 suppliers
$13.50/25G
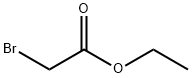
105-36-2
348 suppliers
$5.00/5 g
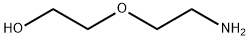
929-06-6
270 suppliers
$10.00/1g

108466-89-3
156 suppliers
$9.00/100mg
Yield:108466-89-3 82%
Reaction Conditions:
Stage #1:di-tert-butyl dicarbonate;2-(2-Aminoethoxy)ethanol in ethanol at 20; for 2 h;Cooling with ice;
Stage #2: with sodium hydride in tetrahydrofuran at 0 - 10; for 0.5 h;
Stage #3:ethyl bromoacetateFurther stages;
Steps:
1-1 Example 1-1:
Using 2-(2-aminoethoxy)ethanol 2 as the starting material (100 mmol, 10.5 g), dissolve 150 mL of ethanol solution, add di-tert-butyl dicarbonate (100 mmol, 23 mL) dropwise under ice bath conditions, and remove the ice Bath, react at room temperature for 2h, distill off the ethanol solvent under reduced pressure; THF is redissolved, add NaH (1.2-1.5eq) at 0 ~ -10 °C, keep this temperature and stir for half an hour, and ethyl bromoacetate 3 (1.2-1.5eq ) Slowly drop the reaction solution, remove the ice bath after the dropwise addition, and stir at room temperature overnight. MeOH was added to the reaction system to make MeOH:THF=1:1, the reaction solution was yellow and clear, and solid NaOH (1-2.2eq) was weighed into the reaction solution, heated to reflux, and reacted for 2h. After the reaction was completed, the solvent was distilled off under reduced pressure. The residue was redissolved with water, extracted twice with ethyl acetate, and the aqueous layer was collected, adjusted to pH 1~3, extracted twice with ethyl acetate, and the organic layer was collected and distilled under reduced pressure to obtain yellow Oily liquid 4. The yield was 82%.
References:
Zhejiang University of Technology;Zhang Fengzhi;Yang Bin CN111269137, 2020, A Location in patent:Paragraph 0077-0079; 072

24424-99-5
821 suppliers
$13.50/25G
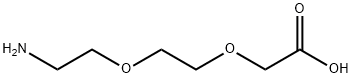
134978-97-5
222 suppliers
$17.00/250mg

108466-89-3
156 suppliers
$9.00/100mg
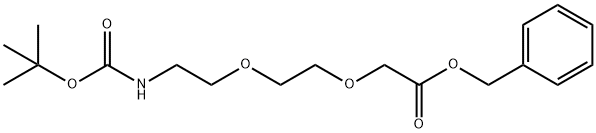
1254054-91-5
0 suppliers
inquiry

108466-89-3
156 suppliers
$9.00/100mg

24424-99-5
821 suppliers
$13.50/25G
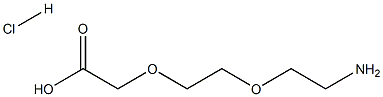
134979-01-4
111 suppliers
$14.00/100mg

108466-89-3
156 suppliers
$9.00/100mg
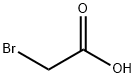
79-08-3
302 suppliers
$9.00/1g
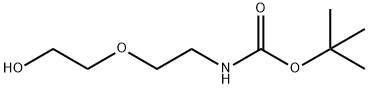
139115-91-6
134 suppliers
$5.00/1g

108466-89-3
156 suppliers
$9.00/100mg