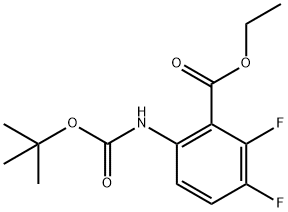
ethyl 6-((tert-butoxycarbonyl)amino)-2,3-difluorobenzoate synthesis
- Product Name:ethyl 6-((tert-butoxycarbonyl)amino)-2,3-difluorobenzoate
- CAS Number:1092513-08-0
- Molecular formula:C14H17F2NO4
- Molecular Weight:301.29

144298-04-4
22 suppliers
inquiry

1092513-08-0
8 suppliers
inquiry
Yield:1092513-08-0 38%
Reaction Conditions:
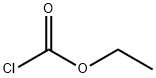
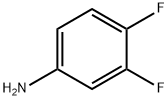
Stage #1: (3,4-difluorophenyl)-carbamic acid-tert-butyl esterwith tert.-butyl lithium in tetrahydrofuran at -78; for 3 h;
Stage #2: with chloroformic acid ethyl ester at -78; for 1 h;
Steps:
44.2 Step-2
To a stirred solution of tert-butyl N-(3,4-difluorophenyl)carbamate (1 g, 4.36 mmol) in THF(30 mL) at -78°C, t-BuLi (7.54 mL, 9.82 mmol) was added dropwise and stirred for 3 h. Ethylchloroformate (0.48 g, 5.1 mmol) was added slowly to the reaction mixture at -78 °C andstirred for 1 h (TLC indicated complete consumption of the starting material). The reaction mixture was brought to 0 °C, treated with saturated aqueous ammonium chloride solution (24mL) over a period of 10 min then warmed to room temperature and diluted with EtOAc (100mL) and water (50 mL). The layers were separated and the aqueous phase was extracted withEtOAc (2 x 30 mL). The combined organic extracts were washed with brine (50 mL), driedover Na2S04, filtered and concentrated under reduced pressure to give the crude residuewhich was purified by column chromatography (100-200 silica gel, 20 g, 2% EtOAc-Hexane)to furnish ethyl 6-(tert-butoxycarbonylamino )-2,3-difluoro-benzoate (0.5 g, 38%) as a whitesolid.1H NMR [400 MHz, CDCh]: 8 9.44 (brs, lH), 8.11-8.08 (m, lH), 7.30-7.23 (m, lH), 4.45(q, J = 6.8 Hz, 2H), 1.51 (s, 9H), 1.41 (t, J = 7.2 Hz, 3H).LCMS: m/z: 202.4 [M+H-lOOt.
References:
WO2018/125961,2018,A1 Location in patent:Page/Page column 110; 111

24424-99-5
824 suppliers
$13.50/25G

1092513-08-0
8 suppliers
inquiry
3863-11-4
330 suppliers
$10.00/25g

1092513-08-0
8 suppliers
inquiry