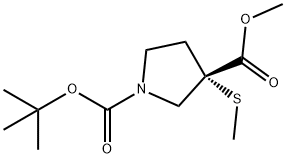
(S)-1-tert-Butyl 3-methyl 3-(methylthio)pyrrolidine-1,3-dicarboxylate synthesis
- Product Name:(S)-1-tert-Butyl 3-methyl 3-(methylthio)pyrrolidine-1,3-dicarboxylate
- CAS Number:1093063-63-8
- Molecular formula:C12H21NO4S
- Molecular Weight:275.36
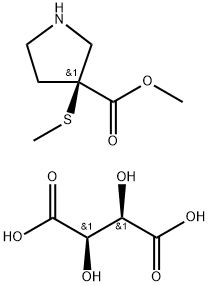
1093063-62-7
13 suppliers
inquiry

24424-99-5
834 suppliers
$13.50/25G

1093063-63-8
1 suppliers
inquiry
Yield:1093063-63-8 100%
Reaction Conditions:
with triethylamine in dichloromethane at 0 - 20;
Steps:
6
Preparation of 3-Methvlsulfanvl-Dvrrolidine-1 ,3-dicarboxvlic acid 1-tert-butvl ester To a cold (O0C) solution of 12BP (28 g, 90.52 mmol) in dry CH2CI2 (250 mL) was added triethylamine (31.5 mL, 226.32 mmol, 2.5 equiv) followed by (BoC)2O (25.7 g, 117.68 mmol, 1.3 equiv). The resulting mixture was stirred from O0C to rt for overnight then diluted with CH2CI2, which was washed with saturated aqueousNaHCO3 solution and brine, dried (MgSO4) and concentrated. Chromatograph on silica gel (hexanes/ethyl acetate, 4:1 ) gave 13BP (23.5 mg, 90.52 mmol, 100%) as a colorless oil.
References:
WO2009/105500,2009,A1 Location in patent:Page/Page column 246
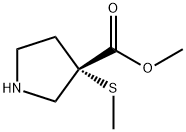
1093063-61-6
3 suppliers
inquiry

24424-99-5
834 suppliers
$13.50/25G

1093063-63-8
1 suppliers
inquiry

942190-28-5
1 suppliers
inquiry

1093063-63-8
1 suppliers
inquiry
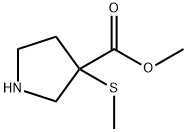
1093063-60-5
4 suppliers
inquiry

1093063-63-8
1 suppliers
inquiry

122684-33-7
231 suppliers
$8.00/1g

1093063-63-8
1 suppliers
inquiry