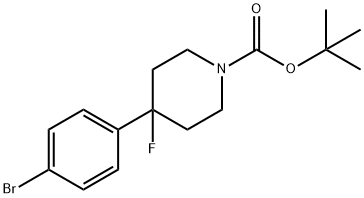
4-(4-Bromophenyl)-4-fluoro-1-piperidinecarboxylic Acid 1,1-Dimethylethyl Ester synthesis
- Product Name:4-(4-Bromophenyl)-4-fluoro-1-piperidinecarboxylic Acid 1,1-Dimethylethyl Ester
- CAS Number:1093064-85-7
- Molecular formula:C16H21BrFNO2
- Molecular Weight:358.25
163209-96-9
36 suppliers
$224.72/1g

1093064-85-7
5 suppliers
inquiry
Yield:1093064-85-7 91%
Reaction Conditions:
with (bis-(2-methoxyethyl)amino)sulfur trufluoride in dichloromethane at -78 - 20;
Steps:
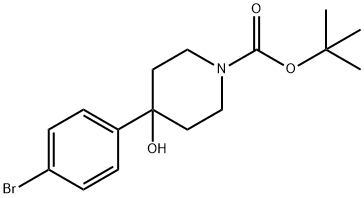
[Bis(2-Methoxyethyl)amino]sulfur trifluoride (2.1 mL, 11.4 mmol) was added to a stirred solution of 4-(4-bromophenyl)-4-hydroxypiperidine-1-carboxylic acid ter/-butyl ester (810 mg, 2.28 mmol) in anhydrous DCM (25 mL), at -78 °C. The reaction mixture was allowed to warm to ambient temperature and stirred for 72 h. The reaction mixture was quenched by the addition of saturated aqueous potassium carbonate (10 mL) and extracted into DCM (3 x 10 mL). The combined organic phase was concentrated in vacuo and the residue purified by flash chromatography (silica, 40 g column, ISCO, 0-100% ethyl acetate in cyclohexane) to afford the title compound as a colourless oil (738 mg, 91%). 1H NMR (CDCl3, 300MHz): 7.50 (d, J = 8.5Hz, 2H), 7.24 (d, J = 8.5Hz, 2H), 4.19-4.05 (m, 2H), 3.29-3.05 (m, 2H), 2.04-1.84 (m, 4H), 1.49 (s, 9H). LCMS (Method B): RT = 4.55 min.
References:
WO2009/151598,2009,A1 Location in patent:Page/Page column 128

24424-99-5
849 suppliers
$13.50/25G

1093064-85-7
5 suppliers
inquiry

