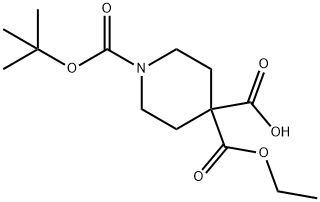
1-(tert-butoxycarbonyl)-4-(ethoxycarbonyl)piperidine-4-carboxylic acid synthesis
- Product Name:1-(tert-butoxycarbonyl)-4-(ethoxycarbonyl)piperidine-4-carboxylic acid
- CAS Number:1093214-64-2
- Molecular formula:C14H23NO6
- Molecular Weight:301.34

124-38-9
132 suppliers
$214.00/14L
142851-03-4
285 suppliers
$5.00/1g

1093214-64-2
17 suppliers
inquiry
Yield:1093214-64-2 31%
Reaction Conditions:
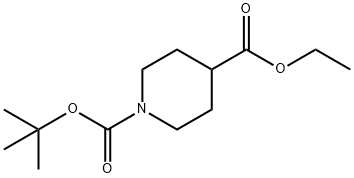
Stage #1: 1-tert-butyl 4-ethyl piperidine-1,4-dicarboxylatewith lithium diisopropyl amide in tetrahydrofuran at -78 - -40;
Stage #2: carbon dioxide in tetrahydrofuran at -78 - 20;
Steps:
38
PREPARATION 38 1-(tert-butoxycarbonyl)-4-(ethoxycarbonyl)piperidine-4-carboxylic acid Lithium diisopropylamide (2.14 ml, 4.28mmol) was added to a solution of N,N,N',N'-tetranethylethylenediamine (1.13 g, 9.72mmol) and piperidine-1,4-dicarboxylic acid 1-tert.butyl 4-ethyl ester (1.0 g, 3.89 mmol) in dry tetrahydrofuran (30 ml) at -78°C. After stirring for 30 minutes the temperature was raised to -40°C. The reaction mixture is again cooled to -78°C and carbon dioxide gas was bubbled through it. The reaction mixture is slowly allowed to come to 0°C, and then stirred at rt for 3 hours. Saturated aqueous ammonium chloride solution (10 ml) was added and the organic layer was separated and concentrated. The residue was treated with an aqueous sodium hydrogen carbonate solution. The aqueous layer was washed with n-hexane, then acidified to pH=5 with 2N HCI and extracted with ethyl acetate, Combined organic layers were dried and concentrated to give 460 mg (31 % yield) of the title product . 1H NMR (400 MHz, DMSO-D6) δ ppm 1.15 (t, J=7.03 Hz, 3 H) 1.36 (s, 9 H) 1.75 - 1.94 (m, 4 H) 3.13 - 3.25 (m, 2 H) 3.31 - 3.45 (m, 2 H) 4.11 (q, J=7.03 Hz, 2 H).
References:
EP2305660,2011,A1 Location in patent:Page/Page column 33-34