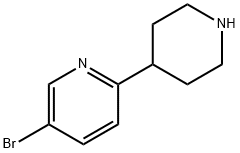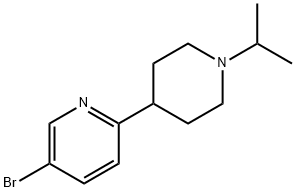
5-Bromo-1'-isopropyl-1',2',3',4',5',6'-hexahydro-[2,4']bipyridinyl synthesis
- Product Name:5-Bromo-1'-isopropyl-1',2',3',4',5',6'-hexahydro-[2,4']bipyridinyl
- CAS Number:1093217-92-5
- Molecular formula:C13H19BrN2
- Molecular Weight:283.21
Yield:1093217-92-5 82%
Reaction Conditions:
Stage #1: 5-bromo-1',2',3',4',5',6'-hexahydro-[2,4']bipyridinyl;acetonewith sodium cyanoborohydride;acetic acid in methanol at 20;
Stage #2: with hydrogenchloride in methanol;water;
Stage #3: in dichloromethane;water; pH=12;
Steps:
5.4
Step 4:5-Bromo-1'-isopropyl-1',2',3',4',5',6'-hexahydro-[2,4']bipyridinyl:5-Bromo-1',2',3',4',5',6'-hexahydro-[2,4']bipyridinyl (6.7 g, 18.9 mmol) was dissolved in 2 % acetic acid in MeOH (40 ml). Propanone (8 ml_, 113 mmol) and Na(CN)BH3 (3.6 g, 57 mmol) were added. The reaction mixture was stirred at RT over night. LC-MS showed only product. The reaction mixture was added 1 N HCI filtered and evaporated in vacuo. The compound was dissolved in water pH was adjusted to PH= 12 extracted with DCM (3x200mL). The DCM phase was dried with Na2CO3 filtered and evaporation gave 4.4g (82%) white crystals. LC-MS (electrospray): m/z: 284 (M+1 ), Rt= 0.89min. 1H NMR (300 MHz, DMSO-D6) δ: 8.6(d, 1 H), 8.45(d,d, 1 H), 7.26(d, 1 H), 2.85(br. D, 2H), 2.65(p, 1 H), 2.6(t, t, 1 H), 2.15(d,t, 2H), 1.78(br. D, 2H), 1.65(m, 2H), 0.98(d, 6H).
References:
WO2008/154126,2008,A1 Location in patent:Page/Page column 44