
6-(tert-ButoxycarbonylaMino)-1H-indole-2-carboxylic acid synthesis
- Product Name:6-(tert-ButoxycarbonylaMino)-1H-indole-2-carboxylic acid
- CAS Number:1093261-27-8
- Molecular formula:C14H16N2O4
- Molecular Weight:276.29

541522-65-0
1 suppliers
inquiry

1093261-27-8
4 suppliers
inquiry
Yield:1093261-27-8 99%
Reaction Conditions:
with water;lithium hydroxide in tetrahydrofuran;methanol at 20;
Steps:
123.C
Lithium hydroxide (0.095 g, 3.96 mmol) was added to a solution of methyl 6-({[(1 ,1- dimethylethyl)oxy]carbonyl}amino)-1 H-indole-2-carboxylate (0.1 15 g, 0.396 mmol) in a 3:1 :1 mixture of THF:methanol:water (2 ml). The reaction mixture was stirred at RT overnight. The solvent was evaporated. The residue was dissolved in water and acidified with 1 N aqueous HCI. The resulting suspension was extracted with ethyl acetate. The organic layer was separated, dried over sodium sulfate and the solvent was evaporated to give the title compound (0.115 g, >99%) as an off-white solid. 1H NMR (400 MHz, DMSOd6): δ ppm 1 1.18 (s, 1 H), 9.21 (s, 1 H), 7.70 (s, 1 H), 7.37 (d, 1 H), 6.93 (dd, 1 H), 6.74 (s, 1 H), 1.46 (s, 9 H). MS: m/z 275 (M-1 ).
References:
WO2008/154271,2008,A1 Location in patent:Page/Page column 137

136818-66-1
55 suppliers
$45.00/50mg

1093261-27-8
4 suppliers
inquiry
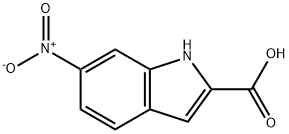
10242-00-9
69 suppliers
$45.00/50mg

1093261-27-8
4 suppliers
inquiry
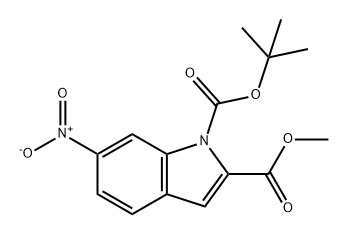
1093261-18-7
0 suppliers
inquiry

1093261-27-8
4 suppliers
inquiry
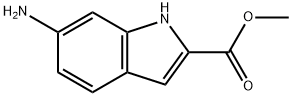
167027-30-7
49 suppliers
inquiry

1093261-27-8
4 suppliers
inquiry