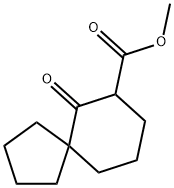
Spiro[4.5]decane-7-carboxylic acid, 6-oxo-, methyl ester synthesis
- Product Name:Spiro[4.5]decane-7-carboxylic acid, 6-oxo-, methyl ester
- CAS Number:1093270-59-7
- Molecular formula:C12H18O3
- Molecular Weight:210.27
![Spiro[4.5]decan-6-one](/CAS/GIF/13388-94-8.gif)
13388-94-8
20 suppliers
$45.00/10mg
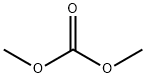
616-38-6
667 suppliers
$5.00/5G
![Spiro[4.5]decane-7-carboxylic acid, 6-oxo-, methyl ester](/CAS/20210111/GIF/1093270-59-7.gif)
1093270-59-7
1 suppliers
inquiry
Yield:1093270-59-7 93%
Reaction Conditions:
with sodium hydride in tetrahydrofuran;Reflux;
Steps:
15.3
Method 3.(E)-1-(7-methylspiro[4.5]dec-6-en-6-yl)but-2-en-1-one. Spiro[4.5]decan-6-one was transformed in three steps into 7-methylspiro[4.5]decan-6-one by methoxycarbonylation (MeOCO2Me, NaH, THF, reflux, 93%) followed by methylation of the resulting ketoester (MeI, K2CO3, acetone, reflux, 2.5 d) and subsequent demethoxycarbonylation of the crude product (AcOH/H2O/H2SO4 10:4:3, reflux, 2 h, 79%). The required intermediate 1-(7-methylspiro[4.5]dec-6-en-6-yl)ethanone was then prepared from 7-methylspiro[4.5]decan-6-one according to the Example 10 via 6-ethynyl-7-methylspiro[4.5]decan-6-ol (lithium acetylide ethylenediamine complex, THF, 2 h, 0-20° C., 75%, 78:22 diastereomeric mixture) and 6-ethynyl-7-methylspiro[4.5]dec-6-ene (POCl3, pyridine, 100° C., 19 h, 45%) that was treated with AcOH/H2SO4 (20° C., 50 min., 34%) to give 1-(7-methylspiro[4.5]dec-6-en-6-yl)ethanone. Subsequent aldol condensation with acetaldehyde (LDA, THF; according to the Example 10) followed by treatment of the crude with acetic anhydride/sodium acetate (80° C., 5 h, 59%; according to the Example 10) gave (E)-1-(7-methylspiro[4.5]dec-6-en-6-yl)but-2-en-1-one.7-methylspiro[4.5]decan-6-oneBoiling point: 57° C. (0.09 mbar)13C-NMR (100 MHz, CDCl3): δ215.63 (s), 56.79 (s, C(5)), 41.98 (d), 40.48 (t), 36.50 (t), 36.39 (t), 34.16 (t), 25.37 (t), 24.81 (t), 22.78 (t), 15.01 (q).6-ethynyl-7-methylspiro[4.5]decan-6-olMS (EI): 192 (1), 191 (1), 177 (9), 166 (5), 164 (5), 163 (10), 159 (22), 151 (40), 149 (23), 145 (15), 135 (77), 131 (21), 125 (12), 121 (26), 117 (21), 110 (73), 108 (63), 97 (50), 95 (100), 93 (66), 91 (49), 82 (86), 81 (72), 79 (61), 77 (41), 67 (98), 55 (71), 53 (74), 41 (75), 39 (43).6-ethynyl-7-methylspiro[4.5]dec-6-ene1H-NMR (400 MHz, CDCl3): δ3.03-3.02 (br. m, H-CC(6)), 2.01 (tm, J=6.2, 0.9, 2H), 1.97-1.86 (m, 1H), 1.90 (m, Me), 1.76-1.53 (m, 7H), 1.47-1.36 (m, 4H).13C-NMR (100 MHz, CDCl3): δ143.25 (s, C(7)), 122.62 (s, C(6)), 82.87 (s, CCH), 80.16 (d, CCH), 45.02 (s, C(5)), 38.70 (t, 2C), 35.41 (t), 31.72 (t), 25.17 (t, 2C), 22.72 (q), 19.75 (t).MS (EI): 174 (44), 159 (22), 146 (12), 145 (36), 132 (100), 131 (42), 117 (81), 115 (42), 105 (20), 103 (23), 91 (67), 79 (13), 77 (24), 67 (9), 65 (13), 53 (11), 51 (12), 41 (16), 39 (16).1-(7-Methylspiro[4.5]dec-6-en-6-yl)ethanone1H-NMR (400 MHz, CDCl3): δ2.29 (s, MeCO), 1.95 (br. t, J=0.6, 6.4, C(8)H2), 1.77-1.56 (m, 8H), 1.60 (t, J=0.9, MeC(7)), 1.52-1.40 (m, 4H).13C-NMR (100 MHz, CDCl3): δ210.41 (s, CO), 142.38 (s), 129.15 (s), 44.62 (s, C(5)), 37.52 (t, 2C), 34.87 (t), 33.61 (q), 30.89 (t), 24.10 (t, 2C), 20.95 (q), 19.37 (t).MS (EI): 192 (3), 177 (9), 163 (7), 159 (2), 150 (18), 149 (100), 135 (16), 121 (7), 107 (17), 93 (18), 91 (18), 81 (13), 79 (15), 77 (12), 67 (9), 55 (8), 43 (32).
References:
US2010/292128,2010,A1 Location in patent:Page/Page column 15-16

108-94-1
527 suppliers
$12.00/50g
![Spiro[4.5]decane-7-carboxylic acid, 6-oxo-, methyl ester](/CAS/20210111/GIF/1093270-59-7.gif)
1093270-59-7
1 suppliers
inquiry