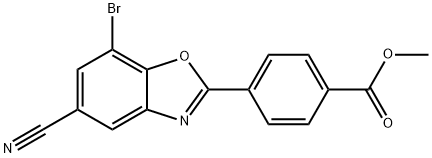
Methyl 4-(7-broMo-5-cyanobenzo[d]oxazol-2-yl)benzoate synthesis
- Product Name:Methyl 4-(7-broMo-5-cyanobenzo[d]oxazol-2-yl)benzoate
- CAS Number:1093395-65-3
- Molecular formula:C16H9BrN2O3
- Molecular Weight:357.16
Yield:1093395-65-3 83%
Reaction Conditions:
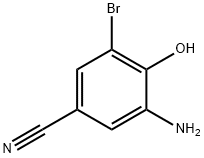
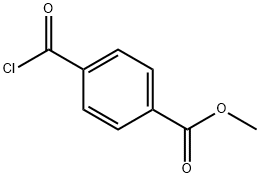
Stage #1: 3-amino-5-bromo-4-hydroxybenzonitrile;4-Methoxycarbonylbenzoyl chloride in 1,4-dioxane;Inert atmosphere;Reflux;
Stage #2: with toluene-4-sulfonic acid in toluene;Reflux;
Steps:
C
To a 3 -L round-bottom flask fitted with a stir bar and a Claisen adapter fitted with a thermocouple and a condenser blanketed with nitrogen was added terephthalic acid monomethyl ester chloride (37.3 g) and a solution of 3-amino-5-bromo-4-hydroxybenzonitrile (40 g, Step B) in dioxane (675 ml). The mixture was heated to reflux and stirred at this temperature overnight. The mixture was then cooled to room temperature and the dioxane was removed in vacuo. The flask was fitted with a Dean-Stark trap and/?-toluenesulfonic acid monohydrate (35.8 g) and toluene (2.5 L) were added. The mixture was heated to reflux and stirred at this temperature overnight. The mixture was then allowed to cool, transferred to a new 5-L flask, and concentrated to a brown solid. The crude product was triturated with methanol to provide 55.5 g (83%) of the title compound.
References:
WO2008/156717,2008,A1 Location in patent:Page/Page column 21
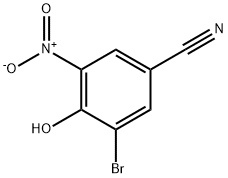
1828-58-6
14 suppliers
inquiry
![Methyl 4-(7-broMo-5-cyanobenzo[d]oxazol-2-yl)benzoate](/CAS/20150408/GIF/1093395-65-3.gif)
1093395-65-3
0 suppliers
inquiry

1689-84-5
142 suppliers
$30.39/250mg
![Methyl 4-(7-broMo-5-cyanobenzo[d]oxazol-2-yl)benzoate](/CAS/20150408/GIF/1093395-65-3.gif)
1093395-65-3
0 suppliers
inquiry