7-tert-butyl 1-methyl 5,6-dihydroimidazo[1,5-a]pyrazine-1,7(8H)-dicarboxylate synthesis
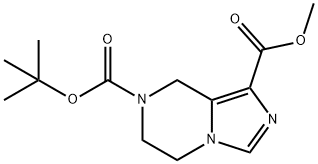
- Product Name:7-tert-butyl 1-methyl 5,6-dihydroimidazo[1,5-a]pyrazine-1,7(8H)-dicarboxylate
- CAS Number:1094091-44-7
- Molecular formula:C13H19N3O4
- Molecular Weight:281.31
![methyl 7-benzyl-5,6,7,8-tetrahydroimidazo[1,5-a]pyrazine-1-carboxylate](/CAS/20180629/GIF/1094091-43-6.gif)
1094091-43-6
1 suppliers
inquiry

24424-99-5
832 suppliers
$13.50/25G
![7-tert-butyl 1-methyl 5,6-dihydroimidazo[1,5-a]pyrazine-1,7(8H)-dicarboxylate](/CAS/20180629/GIF/1094091-44-7.gif)
1094091-44-7
11 suppliers
inquiry
Yield: 83%
Reaction Conditions:
with hydrogen;N-ethyl-N,N-diisopropylamine;palladium(II) hydroxide/carbon in ethanol under 4654.46 Torr; for 48 h;Product distribution / selectivity;
Steps:
1.6
Step 6: Preparation of 7-tert-butyl 1-methyl 5,6-dihydroimidazo[1,5-a]pyrazine-1,7(8H)-dicarboxylate (Intermediate 1G). Under a nitrogen atmosphere, the product Intermediate 1F (3.70 g, 13.64 mmol) was dissolved in ethanol (180 mL) and di-tert-butyldicarbonate (3.87 g, 17.73 mmol) was added followed by DIEA (7.15 mL, 40.9 mmol) and 20% palladium hydroxide on carbon (1.92 g, 2.73 mmol). The resulting black suspension was stirred under a hydrogen atmosphere (90 psi) for 48 hours using a Parr hydrogenator. The mixture was filtered through a pad of celite and washed with methanol. The filtrate was concentrated, dissolved in ethyl acetate and washed with saturated NaHCO3 solution and brine. The organic layer was dried over anhydrous Na2SO4, filtered and concentrated to give the desired product Intermediate 1G as a white solid (3.20 g, 83%) which was used in the next step without further purifications.
References:
Location in patent:Page/Page column 17; 18

85110-06-1
36 suppliers
inquiry
![7-tert-butyl 1-methyl 5,6-dihydroimidazo[1,5-a]pyrazine-1,7(8H)-dicarboxylate](/CAS/20180629/GIF/1094091-44-7.gif)
1094091-44-7
11 suppliers
inquiry