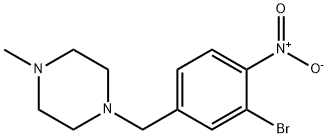
Piperazine, 1-[(3-bromo-4-nitrophenyl)methyl]-4-methyl- synthesis
- Product Name:Piperazine, 1-[(3-bromo-4-nitrophenyl)methyl]-4-methyl-
- CAS Number:1094554-35-4
- Molecular formula:C12H16BrN3O2
- Molecular Weight:314.18

109-01-3
650 suppliers
$5.00/5G
101682-68-2
117 suppliers
$27.00/100mg
![Piperazine, 1-[(3-bromo-4-nitrophenyl)methyl]-4-methyl-](/CAS/20210111/GIF/1094554-35-4.gif)
1094554-35-4
0 suppliers
inquiry
Yield:1094554-35-4 88%
Reaction Conditions:
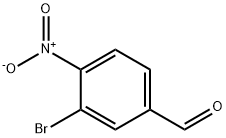
Stage #1: 1-methyl-piperazine;3-bromo-4-nitrobenzaldehydewith acetic acid in toluene at 20; for 1 h;
Stage #2: with sodium tris(acetoxy)borohydride in toluene; for 4 h;
Stage #3: with sodium hydrogencarbonate in methanol;water;toluene;Product distribution / selectivity;
Steps:
N-methyl piperazine (0.95 ml_, 8.56 mmol) was added dropwise to a suspension of 3-bromo-4-nitrobenzaldehyde (0.985 g, 4.28 mmol) and acetic acid (0.29 ml_,5.14 mmol) in toluene (5.5 ml.) at room temperature. The reaction mixture was stirred for 1 h at room temperature before the addition of sodium triacetoxyborohydride (1.36 g, 6.42 mmol). The reaction mixture was stirred for 2 h. Further toluene (2.1 ml.) and sodium triacetoxyborohydride (0.18 g, 0.86 mmol) was added and the reaction mixture was stirred for 2 hours and then quenched by the addition of MeOH (0.99 ml_). The reaction mixture was stirred for 30 min, saturated aqueous NaHCO3 (6.01 ml.) was then added and the reaction mixture was stirred overnight. The phases were separated and the aqueous layer was extracted with toluene (4.0 ml_). The combined organic extracts were concentrated under reduced pressure and purified by flash column chromatography (EtOAc:MeOH:NEt3 9:1 :0.1 ) to give the title compound (1.18 g, 88%) as a low melting point waxy brown solid. δH (400 MHz, CDCI3) 7.81 (1 H, d, J 8.3, Ar H), 7.74 (1 H, d, J 1.5, Ar H), 7.42 (1 H, dd, J 1.7, 8.3, Ar H), 3.53 (2H, s, NCH2Ar), 2.49 (8H, br s, 2 x NCH2CH2), 2.31 (3H, s, NCH3).
References:
WO2009/2916,2008,A2 Location in patent:Page/Page column 27