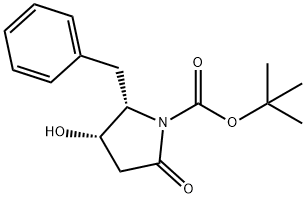
tert-butyl (2S,3S)-2-benzyl-3-hydroxy-5-oxopyrrolidine-1-carboxylate(SALTDATA: FREE) synthesis
- Product Name:tert-butyl (2S,3S)-2-benzyl-3-hydroxy-5-oxopyrrolidine-1-carboxylate(SALTDATA: FREE)
- CAS Number:109579-10-4
- Molecular formula:C16H21NO4
- Molecular Weight:291.34
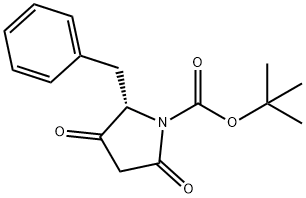
109579-09-1
0 suppliers
inquiry

109579-10-4
13 suppliers
$45.00/10mg
Yield:109579-10-4 441 mg
Reaction Conditions:
with sodium tetrahydroborate in methanol at 0 - 20; for 2 h;
Steps:
4.5.16. (2R,3R) or (2S,3S)-tert-Butyl 2-benzyl-3-hydroxy-5-oxopyrrolidine-1-carboxylate (16a and 16b)
To a solution of 1g (656 mg, 1.61 mmol) was stirred in dry THF (10 mL) at room temperature, and then a solution of TBAF (3.2 mL, 1.0 mol in THF) was dropped. The reaction was stirred for 3 h and quenched with water. The mixture was extracted with EtOAc for three times and the combined organic layers were washed with brine. Dried over anhydrous Na2SO4, filtered, and concentrated, the residue was purified by chromatography on silica gel to give crude intermediate (468 mg), which contain minor impurity of TBAF. To a solution of oxalyl chloride (0.28 mL, 3.26 mmol) in dry CH2Cl2 (10 mL) was dropped DMSO (0.47 mL, 6.53 mmol) over 15 min at -78 °C under argon atmosphere. After being stirred for 30 min, a solution of above crude intermediate (468 mg) in dry CH2Cl2 (5.0 mL) was dropped and the mixture was stirred for anther 1 h, then Et3N (1 mL, 6.96 mmol) was dropped and the mixture was allowed to warm to room temperature. The reaction was quenched with aqueous NaHSO4 solution (1 M, 30 mL), then the organic layer was separated, and the aqueous phase was extracted with CH2Cl2 for two times. The combined organic layers were washed with saturated aqueous solution of NaHSO4, saturated aqueous NaHCO3, and brine. Dried, filtered, and concentrated to give crude product 15a or 15b (457 mg, 92%) as a colorless oil without further purification. The above crude mixture of 15a or 15b was dissolved in MeOH (15 mL) and cooled to 0 °C, then NaBH4 (153 mg, 4.0 mmol) was added in three portions. After being stirred for 2 h at 0 °C to ~room temperature, the reaction was quenched with NaHCO3 aqueous solution and extracted with DCM for three times. The combined organic layers were concentrated and the residue was purified by chromatography on silica gel to give 16a or 16b (441 mg, yield 96%) as a white solid. Mp 98-99 °C; (2S,3S)-16a +27.7 (c 1.12, CHCl3); (2R,3R)-16b -28.4 (c 0.8, CHCl3); IR (film): νmax 3403, 2975, 1770, 1676, 1366, 1287, 1167 cm-1; 1H NMR (400 MHz, CDCl3): δ 7.30-7.19 (m, 5H), 4.51-4.42 (m, 2H), 3.19-3.09 (m, 2H), 2.60 (dd, J=7.2, 17.0 Hz, 1H), 2.40 (dd, J=7.6, 17.0 Hz, 1H), 1.48 (s, 9H) ppm; 13C NMR (100 MHz, CDCl3): δ=171.8, 149.8, 137.8, 129.9, 129.7, 128.6, 126.7, 83.3, 65.6, 62.7, 40.1, 33.9, 28.0 ppm; MS (ESI): 314 (M+Na+); HRMS (ESI) calcd for (C16H21NO4+Na+): 314.1368, found: 314.1372.
References:
Huang, Wei;Ma, Jing-Yi;Yuan, Mu;Xu, Long-Fei;Wei, Bang-Guo [Tetrahedron,2011,vol. 67,# 40,p. 7829 - 7837] Location in patent:experimental part
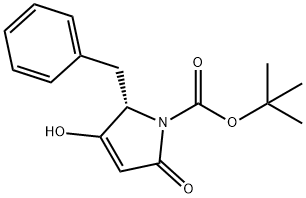
112700-41-1
0 suppliers
inquiry

109579-10-4
13 suppliers
$45.00/10mg

100-39-0
425 suppliers
$10.00/10g

109579-10-4
13 suppliers
$45.00/10mg

87694-53-9
60 suppliers
$33.00/1g

109579-10-4
13 suppliers
$45.00/10mg