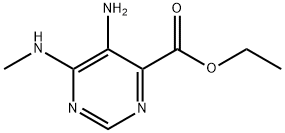
ETHYL 5-AMINO-6-(METHYLAMINO)PYRIMIDINE-4-CARBOXYLATE synthesis
- Product Name:ETHYL 5-AMINO-6-(METHYLAMINO)PYRIMIDINE-4-CARBOXYLATE
- CAS Number:1095823-03-2
- Molecular formula:C8H12N4O2
- Molecular Weight:196.21

64-17-5
706 suppliers
$10.00/50g
201230-82-2
1 suppliers
inquiry

52602-68-3
72 suppliers
$28.00/100mg

1095823-03-2
4 suppliers
inquiry
Yield:1095823-03-2 18%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine;dichloro bis(acetonitrile) palladium(II);2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in acetonitrile at 130; under 6205.94 Torr; for 4 h;
Steps:
21
Synthesis of Compound 21.1. A mixture of compound 11.1 (1.5 g, 9.4 mmol), BINAP (1 g, 2 mmol), bis(acetonitrile)palladium(II) chloride (0.450 g, 1.73 mmol), DIEA (1.97 mL, 11.3 mmol) and EtOH (22 mL) in MeCN (22 mL) was heated (130° C.) under CO (120 psi). After 4 hr, the reaction was filtered through celite, and the solvent was removed in vacuo. The residue was purified by flash chromatography using DCM/EtOAc (SiO2, 100/0 to 0/100) as eluant to afford compound 21.1 (338 mg, 18%) as a yellow solid. 1H NMR (300 MHz, DMSO-d6) δ 7.88 (s, 1H), 7.19 (q, J=4.5 Hz, 1H), 6.43 (br. s., 2H), 4.26 (q, J=7.2 Hz, 2H), 2.91 (d, J=4.5 Hz, 3H), 1.29 (t, J=7.2 Hz, 3H)
References:
US2009/5359,2009,A1 Location in patent:Page/Page column 28