
5-ThiazolecarboxaMide, 2-acetyl-N-[5-chloro-4-(trifluoroMethyl)-2-pyridinyl]- synthesis
- Product Name:5-ThiazolecarboxaMide, 2-acetyl-N-[5-chloro-4-(trifluoroMethyl)-2-pyridinyl]-
- CAS Number:1095825-46-9
- Molecular formula:C12H7ClF3N3O2S
- Molecular Weight:349.72
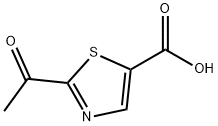
1095824-76-2
73 suppliers
$90.00/100mg
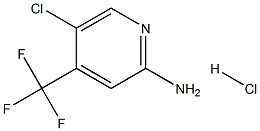
1095824-77-3
41 suppliers
$35.00/250mg
![5-ThiazolecarboxaMide, 2-acetyl-N-[5-chloro-4-(trifluoroMethyl)-2-pyridinyl]-](/CAS/GIF/1095825-46-9.gif)
1095825-46-9
14 suppliers
inquiry
Yield:1095825-46-9 96%
Reaction Conditions:
Stage #1: 2-acetyl-1,3-thiazole-5-carboxylic acidwith oxalyl dichloride;N,N-dimethyl-formamide in 1,2-dimethoxyethane at 10 - 35; for 2 h;
Stage #2: 5-chloro-4-(trifluoromethyl)pyridin-2-amine hydrochloridewith pyridine in acetonitrile at 5 - 50; for 2 h;
Steps:
1 Synthesis of 2-acetyl-N-(5-chloro-4-(trifluoromethyl)pyridin-2-yl)-1,3-thiazole-5-carboxamide
2-Acetyl-1,3-thiazole-5-carboxylic acid (24.7 g), 1,2-dimethoxyethane (43 mL), N,N-dimethylformamide (0.047 g) and oxalyl chloride (17.8 g) were put into a four-necked eggplant flask (100 mL), and the mixture was stirred at room temperature for 2 hr (Reaction Solution 1).
Separately, 5-chloro-4-(trifluoromethyl)pyridin-2-amine hydrochloride (30.0 g), acetonitrile (96 mL) and pyridine (30.8 g) were put into a four-necked eggplant flask (500 mL), and the mixture was cooled to about 5° C. (Reaction Solution 2).
Reaction Solution 1 was added to Reaction Solution 2 at 35° C. or lower, and 1,2-dimethoxyethane (15 mL) was added thereto.
The mixture was stirred at room temperature for 1 hr, warmed to about 50° C., and stirred for 1 hr.
Water (210 mL) was added thereto at about 50° C., and the mixture was cooled to room temperature, and was stirred for 30 min.
The crystallized substance was collected by filtration, washed with a mixed solvent of acetonitrile (22.5 mL) and water (67.5 mL), washed with water (180 mL), and dried under reduced pressure at 50° C. to give the title compound. pale-brown powder, 43.4 g, yield 96%, purity 99.0% (HPLC).
References:
US2020/317659,2020,A1 Location in patent:Paragraph 0432; 0564-0567

1095824-76-2
73 suppliers
$90.00/100mg
![5-ThiazolecarboxaMide, 2-acetyl-N-[5-chloro-4-(trifluoroMethyl)-2-pyridinyl]-](/CAS/GIF/1095825-46-9.gif)
1095825-46-9
14 suppliers
inquiry