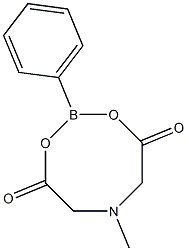
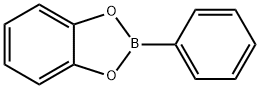
2-Phenyl-6-methyl-1,3,6,2-dioxazaborocane-4,8-dione synthesis
- Product Name:2-Phenyl-6-methyl-1,3,6,2-dioxazaborocane-4,8-dione
- CAS Number:109674-45-5
- Molecular formula:C11H12BNO4
- Molecular Weight:233.0283

108-86-1
497 suppliers
$10.00/5g

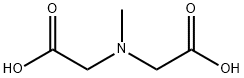
5419-55-6
371 suppliers
$12.00/5g
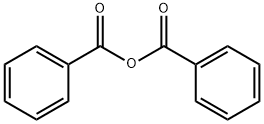
4408-64-4
179 suppliers
$6.00/5g

109674-45-5
19 suppliers
inquiry
Yield:109674-45-5 81%
Reaction Conditions:
Stage #1: bromobenzene;Triisopropyl boratewith n-butyllithium in tetrahydrofuran at -78 - 20; for 0.75 h;
Stage #2: N-methyliminodiacetic acid in tetrahydrofuran;dimethyl sulfoxide; for 3 h;Heating / reflux;
Steps:
20
Example 20; Preparation of Protected Organoboronic Acids Without the Formation of the Corresponding Free Boronic Acid; Phenyl-MIDA-Boronate (304); To a dry 100 mL Schlenk flask equipped with a stir bar, fitted with a rubber septum, and placed under Ar atmosphere, was added THF (25 mL), bromoboenzene (2.0 mL, 19 mmol) and triisopropyl borate (5.3 mL, 23 mmol). The stirred solution was cooled to -78° C. To the solution was added n-BuLi (9.1 mL, 2.5 M, 23 mmol). The pale orange solution was stirred for 15 min. The solution was allowed to warm to room temperature with stirring for 30 min. To the solution was added DMSO (15 mL) and N-methyliminodiacetic acid (8.39 g, 57.1 mmol). The flask was fitted with a distillation apparatus. The mixture was brought to reflux, and as solvent was distilled the distillation pot was periodically charged with toluene to maintain a constant volume. The crude reaction mixture was concentrated in vacuo to afford an off-white solid. To the flask was added acetone (200 mL). The resulting suspension was filtered through a thin pad of celite. The filtrate was concentrated in vacuo. The resulting residue was adsorbed onto Florisil gel. This powder was dry-loaded onto a silica gel column slurry packed in Et2O. The column was flushed with Et2O (approximately 400 mL), then was eluted with Et2O:MeCN (5:1) to afford 304 as a colorless solid, 3.592 g (81%).
References:
US2009/30238,2009,A1 Location in patent:Page/Page column 23
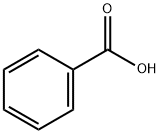
93-97-0
366 suppliers
$6.00/25g

109674-45-5
19 suppliers
inquiry
65-85-0
1330 suppliers
$10.00/25g

109674-45-5
19 suppliers
inquiry

4408-64-4
179 suppliers
$6.00/5g
5747-23-9
50 suppliers
$155.00/50mg

109674-45-5
19 suppliers
inquiry
