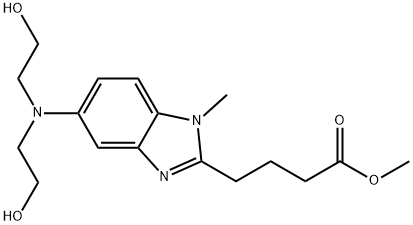
5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid methyl ester synthesis
- Product Name:5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid methyl ester
- CAS Number:109882-31-7
- Molecular formula:C17H25N3O4
- Molecular Weight:335.4
Yield:109882-31-7 24.6 g
Reaction Conditions:
Stage #1: methanol;C16H25N3O5with hydrogenchloride in acetic acid methyl ester;water at 73; for 2.5 h;
Stage #2: with ammonia in acetic acid methyl ester;water; pH=6.0;
Steps:
2 Example 2
A 300 ml, pressure reactor equipped with a magnetic stirring bar was charged with the compound of formula [12] (19.54 g, 0.08 mol), boric acid (1.48 g, 24 mmol), and methylamine aqueous solution (35 ml, 0.4 mol). The mixture was stirred at 800 rpm at 70 °C in the sealed reactor for 6.0 h. The mixture was concentrated on a rotary evaporator at 75 °C at 700 mbar and co-evaporated with methanol (150) ml three times. The evaporation residue was diluted with 150 ml of methanol and transferred into a pressure reactor containing palladium on activated carbon (4.37 g, 1.6 mmol, 3.9 % wet basis) moistened with water (1 ml). The reactor was flushed with nitrogen three times, with hydrogen four times, and pressurized to 50 psi with hydrogen. The reaction mixture was stirred at 900 rpm at 23 °C for 2.5 h under hydrogen atmosphere of 50-55 psi. The reactor was charged with a solution of glutaric anhydride (12.36 g, 0.1 mol, 96 ) in methyl acetate (30 ml) via a syringe over 0.1 h and the mixture was stirred at 1000 rpm at 23 °C for 2.0 h under nitrogen atmosphere. The mixture was filtered over a sintered glass filter and the filtrate was collected in a 500 ml, round bottomed flask. The flask was equipped with a magnetic stirring bar as well as a reflux condenser and charged with hydrochloric acid (16.11 ml, 0.18 mol, 35 ). The reaction mixture was stirred at 700 rpm at 73 °C for 2.5 h and cooled to room temperature over 1.0 h. The pH of the mixture was adjusted with concentrated aqueous ammonia solution to the value of 6.0 and the mixture was concentrated at 60 °C at 350-100 mbar on the rotary evaporator. The residue was diluted with water (150 ml) and washed with dichloromethane (200 ml). The pH of the aqueous layer was adjusted with concentrated aqueous ammonia solution to the value of 8.5 and the layer was extracted with dichloromethane (200 ml). The extract was concentrated at 60 °C at 950 mbar to one fifth of initial volume and diluted with ethyl acetate (200 ml). The solution was filtered over a sintered glass filter and the filtrate wasevaporated at 60 °C at 450-100 mbar to produce [11, R = Me] (24.6 g) in an overall isolated yield of 92%. Characteriz ation XH NMR (400 MHz, DMSO-J<5): δ (ppm) = 2.04 (q, J = 7.33 Hz, 2H), 2.48 (t, J = 7.33 Hz, 2H), 2.82 (t, J = 7.38 Hz, 2H), 3.41 (t, J = 6.32 Hz, 4H), 3.58 (t, J = 6.34 Hz, 4H), 3.61 (s, 3H), 3.64 (s, 3H), 4.57 (s, b, 2H), 6.75 (dd, Ji = 2.35 Hz, J2 = 8.81 Hz, 1H), 6.89 (d, J = 2.27 Hz, 1H), 7.22 (d, J = 8.80 Hz, 1H).
References:
WO2013/150020,2013,A1 Location in patent:Page/Page column 17-18
3543-75-7
398 suppliers
$35.00/10mg
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid methyl ester](/CAS/GIF/109882-31-7.gif)
109882-31-7
15 suppliers
inquiry
109882-29-3
17 suppliers
inquiry
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid methyl ester](/CAS/GIF/109882-31-7.gif)
109882-31-7
15 suppliers
inquiry
![2,2'-[(4-fluoro-3-nitrophenyl)imino]bisethanol](/CAS/GIF/29705-38-2.gif)
29705-38-2
41 suppliers
inquiry
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid methyl ester](/CAS/GIF/109882-31-7.gif)
109882-31-7
15 suppliers
inquiry
![2,2'-[[4-(methylamino)-3-nitrophenyl]imino]bisethanol](/CAS/GIF/2784-94-3.gif)
2784-94-3
4 suppliers
inquiry
![5-[Bis(2-hydroxyethyl)amino]-1-methyl-1H-benzimidazole-2-butanoic acid methyl ester](/CAS/GIF/109882-31-7.gif)
109882-31-7
15 suppliers
inquiry

![1H-Benzimidazole-2-butanoic acid, 5-[bis(2-hydroxyethyl)amino]-2,3-dihydro-2-hydroxy-1-methyl-](/CAS/20210305/GIF/1464999-05-0.gif)