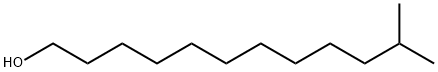
11-methyldodecanol synthesis
- Product Name:11-methyldodecanol
- CAS Number:85763-57-1
- Molecular formula:C13H28O
- Molecular Weight:200.36
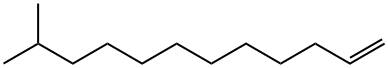
106787-53-5
0 suppliers
inquiry

85763-57-1
15 suppliers
$145.00/100mg
Yield: 95%
Reaction Conditions:
Stage #1:11-methyl-dodec-1-ene with sodium tetrahydroborate;iodine in tetrahydrofuran at 0 - 20; for 3 h;Inert atmosphere;
Stage #2: with dihydrogen peroxide;sodium hydroxide in tetrahydrofuran;water at 0;
Steps:
11-Methyldodecan-1-ol (12)
A solution of I2 (13.8 g, 54.3 mmol) in dry THF (148 mL) was added to a solution of NaBH4 (1.87 g, 49.4 mmol) in THF (148 mL) and the brown solution was heated at reflux under a dry nitrogen atmosphere for 40 min, and the resulting cloudy and colorless solution was cooled to 0 °C. A solution of 11 (18.0 g, 98.7 mmol) in dry THF (10.0 mL) was added and the resulting reaction mixture was warmed to rt and stirred under nitrogen for 3 h. The reaction mixture was cooled to 0 °C, then 30% aq H2O2/3 N aq NaOH (1:1, 8 × 50 mL aliquots) were separately added to the reaction mixture, with each addition resulting in a strongly exothermic reaction and production of a large volume of gas. After cooling, the mixture was concentrated in vacuo, then diluted into water and extracted with Et2O (4 × 125 mL). The combined organic phases were washed with brine (2 × 150 mL), then dried (MgSO4), filtered and evaporated in vacuo to give a yellow liquid. Purification by flash chromatography (Et2O/hexanes, 20 → 40%) afforded 12 as a colorless liquid (18.8 g, 95%); 1H NMR (500 MHz, CDCl3) δ 0.86 (6H, d, J = 6.6 Hz), 1.10-1.18 (2H, m), 1.21-1.38 (15H, m), 1.46-1.59 (3H, m), 3.63 (2H, t, J = 6.7 Hz); 13C NMR (125 MHz, CDCl3) δ 22.8, 25.9, 27.6,28.1, 29.6, 29.8, 29.8, 29.8, 30.1, 32.9, 39.2, 63.2; IR 1466, 2853, 2923 cm-1; HRMS (ESI+) calcd for C13H28NaO [M + Na]+ m/z 223.2032, found 223.2032.
References:
Richardson, Mark B.;Williams, Spencer J. [Beilstein Journal of Organic Chemistry,2013,vol. 9,p. 1807 - 1812] Location in patent:supporting information

53463-68-6
203 suppliers
$6.00/1g
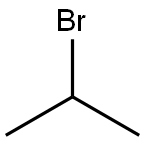
75-26-3
452 suppliers
$10.00/10g

85763-57-1
15 suppliers
$145.00/100mg
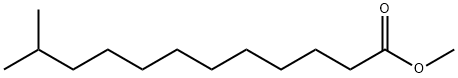
5129-57-7
21 suppliers
$39.00/1mg

85763-57-1
15 suppliers
$145.00/100mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

85763-57-1
15 suppliers
$145.00/100mg

629-41-4
357 suppliers
$9.00/10g

85763-57-1
15 suppliers
$145.00/100mg