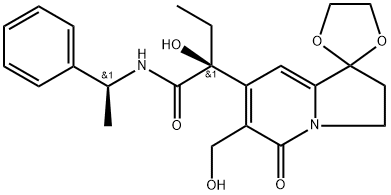
(S)-α-ethyl-α-hydroxy-1,1-(ethylenedioxy)-6-hydroxymethyl-5-oxo-1,2,3,5-tetrahydroindolizine-7-[N-(1S)-1-phenylethyl]acetamide synthesis
- Product Name:(S)-α-ethyl-α-hydroxy-1,1-(ethylenedioxy)-6-hydroxymethyl-5-oxo-1,2,3,5-tetrahydroindolizine-7-[N-(1S)-1-phenylethyl]acetamide
- CAS Number:110314-10-8
- Molecular formula:C23H28N2O6
- Molecular Weight:428.49
Yield:-
Reaction Conditions:
in dichloromethane;benzene;
Steps:
6.1 (1)
(1) (S)-α-Ethyl-1,1-(ethylenedioxy)-α-hydroxy-6-(hydroxymethyl)-N-[(S)-1-phenylethyl]-5-oxo-1,2,3,5-tetrahydroindolizine-7-acetamide (VII) Added to 3 ml of S-(-)-α-methylbenzylamine was 1 g of the compound (Ib) obtained in Example 2 and the resulting mixture was stirred at 100° C. for 18 hours under a nitrogen gas stream. After completion of the reaction, 150 ml of dichloromethane was added and the resulting mixture was washed first with 10% citric acid and then with water. The mixture was then dried over anhydrous sodium sulfate and the solvent was thereafter distilled off. The residue was purified by column chromatography on silica gel (30 g). From a 98:2 elude of chloroform and methanol, 1 g of a colorless oily substance was obtained. The oily substance was recrystallized from a mixed solvent of dichloromethane and n-hexane. Then, 900 mg of colorless crystals were obtained by filtration. The thus-obtained crystals were dissolved in hot benzene and the resultant benzene solution was allowed to stand overnight. Precipitated crystals were collected by filtration to obtain 460 mg of colorless needle-like crystals. The crystals were recrystallized again from a mixed solvent of dichloromethane and n-hexane, thereby obtaining 380 mg of the title compound as colorless needle-like crystals. Melting point: 115°-116° C. NMR (CDCl3) δ: 0.88(3H, t, J=7 Hz, CH3 CH2 --), 1.50(3H, d, J=7 Hz, CH3 CH--), 2.32(2H, t, J=7 Hz, C2 --H), 4.10(6H, m, --O--(CH2)2 --O, C3 --H), 4.88(2H, ABq, J=12.6 Hz, CH2 OH), 5.10(1H, m, CHCH3), 6.67(1H, s, C8 --H) 7.32(5H, s, Ph). IR νmaxKBr cm-1: 3400, 1740, 1650, 1585. Elemental analysis: Calculated for C23 H28 N2 O4.1/4H2 O: C, 63.80; H, 6.63; N, 6.47. Found: C, 63.51; H, 6.69; N, 6.45.
References:
US4778891,1988,A
2627-86-3
541 suppliers
$10.60/1gm:
![Spiro[1,3-dioxolane-2,6'(3'H)-[1H]pyrano[3,4-f]indolizine]-3',10'(4'H)-dione, 4'-ethyl-7',8'-dihydro-4'-hydroxy-](/CAS/20200611/GIF/102978-41-6.gif)
102978-41-6
7 suppliers
inquiry
![Spiro[1,3-dioxolane-2,1'(5'H)-indolizine]-7'-acetamide, α-ethyl-2',3'-dihydro-α-hydroxy-6'-(hydroxymethyl)-5'-oxo-N-(1-phenylethyl)-, [R-(R*,S*)]- (9CI)](/CAS/20210305/GIF/127318-98-3.gif)
127318-98-3
1 suppliers
inquiry
![(S)-α-ethyl-α-hydroxy-1,1-(ethylenedioxy)-6-hydroxymethyl-5-oxo-1,2,3,5-tetrahydroindolizine-7-[N-(1S)-1-phenylethyl]acetamide](/CAS/20211123/GIF/110314-10-8.gif)
110314-10-8
5 suppliers
inquiry