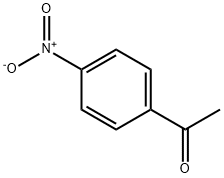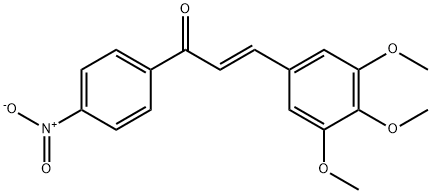
(2E)-1-(4-nitrophenyl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one synthesis
- Product Name:(2E)-1-(4-nitrophenyl)-3-(3,4,5-trimethoxyphenyl)prop-2-en-1-one
- CAS Number:1103585-01-8
- Molecular formula:C18H17NO6
- Molecular Weight:343.33
Yield:1103585-01-8 84.68%
Reaction Conditions:
with sodium hydroxide in methanol;lithium hydroxide monohydrate at 20;Claisen-Schmidt Condensation;
Steps:
Synthetic procedure
General procedure: The reaction mixture of para-substituted acetophenone (0.01 M) and subsequentaldehyde (0.01 M) was stirred for 2-3 h in methanol (30 ml) in a round bottom flask.NaOH solution (10 ml sodium hydroxide solution 40%) was added drop wise andcontinuous stirring at room temperature. The reaction mixture was placed at roomtemperature overnight and then it was poured into ice cold water after that the solutionwas acidified by dilute HCl (hydrochloric acid). Then the precipitated of finalproduct was filtered, desiccated and recrystallized from ethanol (Rectified spirit)[27].
References:
Narwal, Sangeeta;Kumar, Sanjiv;Verma, Prabhakar Kumar [Research on Chemical Intermediates,2021,vol. 47,# 4,p. 1625 - 1641]