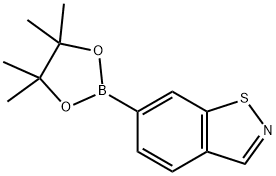
6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]isothiazole synthesis
- Product Name:6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]isothiazole
- CAS Number:1104071-55-7
- Molecular formula:C13H16BNO2S
- Molecular Weight:261.15
Yield:1104071-55-7 56%
Reaction Conditions:
with potassium acetate;tricyclohexylphosphine;tris-(dibenzylideneacetone)dipalladium(0) in 1,4-dioxane at 110; for 0.5 h;Sealed tube;Microwave irradiation;
Steps:
42
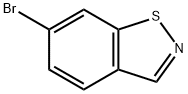
6-(4,4,5,5-Tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]isothiazole. To a mixture of 6-bromobenzo[d]isothiazole (0.86 g, 4.0 mmol)(prepared as described in WO 2008/036308), potassium acetate (0.38 mL, 6.0 mmol), bis(pinacolato)diboron (1.3 g, 5.2 mmol), tris(dibenzylideneacetone)dipalladium (0) (0.18 g, 0.20 mmol), and tricyclohexylphosphine (0.12 g, 0.44 mmol) was added dioxane (5 mL). The resulting mixture was sealed and heated at 1 10°C for 30 minutes under microwave irradiation. The mixture was cooled and passed through a short path of Celite, washing with DCM (3 x 10 mL). The combined organic phases were concentrated to give a residue that was purified by chromatography on silica gel (hexanes - 50 % EtOAc in hexanes) and triturat on with hexanes provided the product as a white powder (0.59 g, 56 %). LCMS (API-ES) m/z (%): 230.2 (100 %, M+H+); 1H NMR (400 MHz, CDCl3) δ ppm 8.95 (s, 1 H) 8.58 (s, 1 H) 7.91 - 8.02 (m, 2 H) 1.41 (s, 12 H).
References:
WO2009/11871,2009,A2 Location in patent:Page/Page column 185