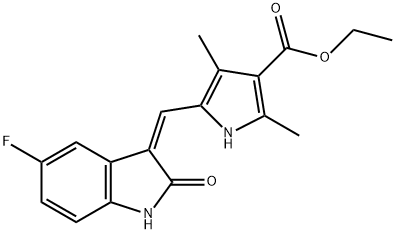
(Z)-ethyl 5-((5-fluoro-2-oxoindolin-3-ylidene)Methyl)-2,4-diMethyl-1H-pyrrole-3-carboxylate synthesis
- Product Name:(Z)-ethyl 5-((5-fluoro-2-oxoindolin-3-ylidene)Methyl)-2,4-diMethyl-1H-pyrrole-3-carboxylate
- CAS Number:1104253-05-5
- Molecular formula:C18H17FN2O3
- Molecular Weight:328.34

2199-51-1
245 suppliers
$7.00/250mg
![1H-Indole, 5-fluoro-1-(trimethylsilyl)-2-[(trimethylsilyl)oxy]-](/CAS/20200611/GIF/1374685-40-1.gif)
1374685-40-1
1 suppliers
inquiry

1104253-05-5
22 suppliers
inquiry
Yield:1104253-05-5 40%
Reaction Conditions:
Stage #1: 2,4-dimethyl-1H-pyrrole-3-carboxylic acid ethyl esterwith Vilsmeier reagent in acetonitrile at 20; for 1 h;
Stage #2: 5-fluoro-1-(trimethylsilyl)-2-(trimethylsilyloxy)-1H-indole in acetonitrile at 20; for 2.58333 h;
Steps:
21 Example 21 Preparing a Sunitinib Analogue 17 by Vilsmeier Salt and (2,4-dimethyl)-1H-pyrrole-3-carboxylic acid ethyl ester (40)
A mixture of 8 (1.14 g, 1.0 eq), (NH4)2SO4 (0.10 g, 0.1 eq.) and 14.1 mL (9 eq.) of HMDS was heated to reflux for 7 h to give a clear solution, which was distilled at 60° C. under vacuum to remove HMDS providing 20.
To the ice cooled mixture of DMF (3.07 g) and DCM (50 mL) was slowly added oxalyl chloride (5.23 mL).
A white slurry formed and which was stirred in an ice bath for 40 min.
The DCM was evaporated at r.t. under reduced pressure to give a semi-solid which was dried at 60° C. in vacuo for 30 min to give the Vilsmeier salt 41 as a white powder.
To a slurry of the Vilsmeier salt (1.1 g) 41 and MeCN (10.8 mL) was added (2,4-dimethyl)-1H-pyrrole-3-carboxylic acid ethyl ester (40) in MeCN (14.9 mL) dropwise over a 20 min period providing a clear red-brown solution followed by the precipitation of a white solid.
The resultant slurry was stirred at r.t. for 40 min and then 20 was added into the reaction mixture providing a clear dark red-brown coloured solution.
After about five minutes a yellow solid precipitated which was stirred at r.t. for a further 2.5 h.
The reaction product was collected by filtration and the filter cake was washed three times with MeCN (5 mL each) and dried under vacuum to give 1.05 g (40%) of (Z)-ethyl 5-((5-fluoro-2-oxoindolin-3-ylidene)methyl)-2,4-dimethyl-1H-pyrrole-3-carboxylate (17) as a yellow solid with 93.4% HPLC purity. 1H NMR (300 MHz, DMSO-d6) δ 13.84 (s, 1H, H17), 10.91 (s, 1H, H7), 7.72, (dd, J=9.4, 2.5 Hz, 1H, H3), 7.67 (s, 1H, H10), 6.93-6.87 (m, 1H, H6), 6.79 (dd, J=8.5, 4.6 Hz, 1H, H2), 4.14 (q, J=7.1 Hz, 2H, H22), 2.47 (s, 3H, H24), 2.44 (s, 3H, H25), 1.24 (t, J=7.1 Hz, 3H, H23); 13C NMR (300 MHz, DMSO-d6) δ 170 (C8), 164 (C19) 159 (C1), 141 (C4), 135 (C13), 133 (C11), 127 (C5), 126 (C3), 125 (C10), 116 (C15), 114 (C9), 113 (C6), 110 (C14), 107 (C2), 59 (C22), 16(C24), 14(C25), 12 (C23); ESI-MS (Positive mode): 329 ([MH]+, 67%); ESI-MS (Negative mode): 327 ([M-H]+, 100%).
References:
US2013/190512,2013,A1 Location in patent:Paragraph 0094

56341-41-4
520 suppliers
$12.00/5g

1104253-05-5
22 suppliers
inquiry