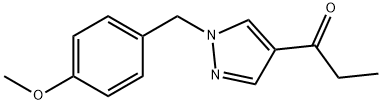
1-Propanone,1-[1-[(4-Methoxyphenyl)Methyl]-1H-pyrazol-4-yl]- synthesis
- Product Name:1-Propanone,1-[1-[(4-Methoxyphenyl)Methyl]-1H-pyrazol-4-yl]-
- CAS Number:1105039-60-8
- Molecular formula:C14H16N2O2
- Molecular Weight:244.29
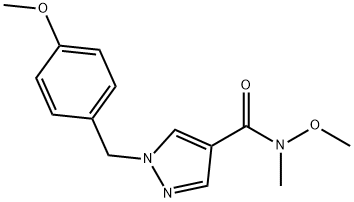
1105039-59-5
7 suppliers
inquiry
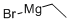
925-90-6
347 suppliers
$12.00/5ml
![1-Propanone,1-[1-[(4-Methoxyphenyl)Methyl]-1H-pyrazol-4-yl]-](/CAS/GIF/1105039-60-8.gif)
1105039-60-8
13 suppliers
inquiry
Yield:1105039-60-8 97%
Reaction Conditions:
in tetrahydrofuran at 20 - 50; for 3.5 h;
Steps:
5
l-(l-(4-Methoxybenzyl)-lH-pyrazol-4-yl)propan-l-oneAccording to Scheme 3 Step 2: Ethylmagnesium bromide (3ν, 37.2 mmol, 12.4 mL) was added dropwise at room temperature to a solution of N-methoxy- 1 -(4- methoxybenzyl)-N-methyl-lH-pyrazole-4-carboxamide (33.8 mmol, 9.30 g) in TηF (80 mL) and the reaction mixture was stirred for 1 hour. Then ethylmagnesium bromide (3N, 37.2 mmol, 12.4 mL) was added and the reaction mixture was stirred for 1 hour.
References:
WO2009/10455,2009,A2 Location in patent:Page/Page column 53-54