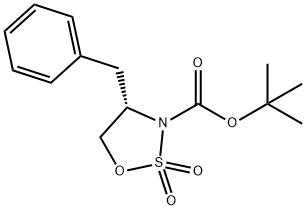
(S)-3-Boc-4-benzyl-1,2,3-oxathiazolidine 2,2-dioxide synthesis
- Product Name:(S)-3-Boc-4-benzyl-1,2,3-oxathiazolidine 2,2-dioxide
- CAS Number:1105712-07-9
- Molecular formula:C14H19NO5S
- Molecular Weight:313.37
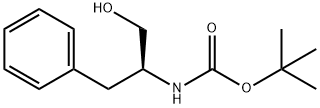
66605-57-0
316 suppliers
$6.00/1g

1105712-07-9
10 suppliers
inquiry
Yield:1105712-07-9 92%
Reaction Conditions:
Stage #1: (S)-N-tert-butoxycarbonyl-2-amino-3-phenylpropanolwith triethylamine in dichloromethane at 0; for 0.0833333 h;Inert atmosphere;
Stage #2: with sulfuryl dichloride in dichloromethane at -20; for 0.25 h;Inert atmosphere;
Steps:
General procedure for the cyclization of N-Boc derivativesβ-amino alcohols 4(a-c)
General procedure: A solution of β-amino alcohols (1 mmol) in dry dichloromethane (5 mL) and triethylamine (5 mmol) were taken in 50 mL round bottom ask under nitrogen and the solution was cooled to 0 °C over 5 min. A solution of sulfurylchloride (2 mmol) was added dropwise at -20 °C over 15 min. After completion of the reaction, the organic layer was extracted with ethyl acetate (3 × 5 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude product is purifed over silica gel column chromatography eluted with (DCM-MeOH-9.5:0.5) to give yellow oil.
References:
K'tir, Hacène;Aouf, Zineb;Souk, Tan Otea;Zerrouki, Rachida;Berredjem, Malika;Aouf, Nour-Eddine [Phosphorus, Sulfur and Silicon and the Related Elements,2017,vol. 192,# 5,p. 555 - 559]
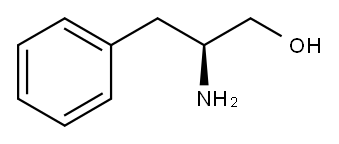
3182-95-4
458 suppliers
$5.00/5g

1105712-07-9
10 suppliers
inquiry

24424-99-5
821 suppliers
$13.50/25G

1105712-07-9
10 suppliers
inquiry
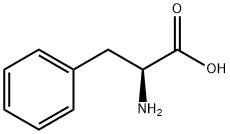
63-91-2
876 suppliers
$10.00/100G

1105712-07-9
10 suppliers
inquiry