6-benzyl-2-(trifluoroMethyl)-5,6,7,8- tetrahydro-1,6-naphthyridine synthesis
- Product Name:6-benzyl-2-(trifluoroMethyl)-5,6,7,8- tetrahydro-1,6-naphthyridine
- CAS Number:1108192-98-8
- Molecular formula:C16H15F3N2
- Molecular Weight:292.3
Yield:1108192-98-8 21.3 g
Reaction Conditions:
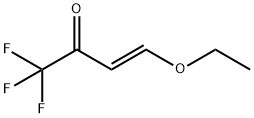
Stage #1: (E)-4-Ethoxy-1,1,1-trifluoro-3-buten-2-one;1-Benzyl-4-(1-pyrrolidinyl)-1,2,3,6-tetrahydro-pyridine in 1,4-dioxane at 20;
Stage #2: with ammonium acetate for 3 h;Reflux;
Steps:
2.2 (2) Synthesis of 6-benzyl-2-trifluoromethyl-5,6,7,8-tetrahydro-1,6-naphthyridine
A solution of 48 g (0.2 mol) of 1-benzyl-4- (pyrrole-1-yl) -1,2,3,6-tetrahydropyridine (Example 1- (1) , 4-dioxane, cooled to 10 ° C, and 33.6 g (0. 2 mol) of (Ε) -4-ethoxy-1,1,1-trifluoro-3 Butene-2-one and 50 mL of dioxane was added dropwise over 20 minutes, and the mixture was stirred at room temperature overnight. (0.44 mol) of ammonium acetate was added and heated to reflux for 3 hours. The mixture was cooled to room temperature and dissolved under reduced pressure. The residue was dissolved in 150 mL of diethyl ether, washed with water (200 mL), saturated brine (200 mL) Washed with anhydrous magnesium sulfate, concentrated under reduced pressure, and then purified by column chromatography (ethyl acetate: petroleum ether = 1: 10) to give pale yellow solid 6-benzyl-2-trifluoromethyl-5,6,6 , 8-tetrahydro-1,6-naphthyridine 21. 3g, m.p. 66. 2 ° C.
References:
CN106467537,2017,A Location in patent:Paragraph 0165; 0166; 0169; 0170