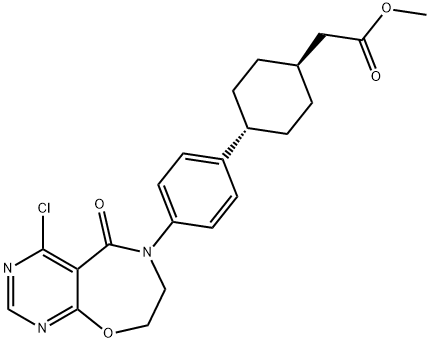
Methyl 2-((1r,4r)-4-(4-(4-aMino-5-oxo-7,8-dihydropyriMido[5,4-f][1,4]oxazepin-6(5H)-yl)phenyl)cyclohexyl)acetate synthesis
- Product Name:Methyl 2-((1r,4r)-4-(4-(4-aMino-5-oxo-7,8-dihydropyriMido[5,4-f][1,4]oxazepin-6(5H)-yl)phenyl)cyclohexyl)acetate
- CAS Number:1109277-49-7
- Molecular formula:C22H24ClN3O4
- Molecular Weight:429.9
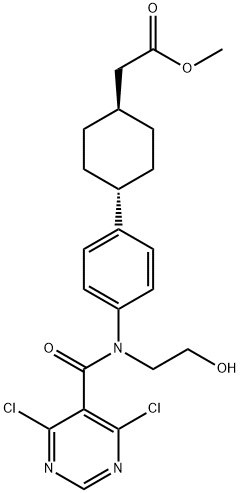
1109277-52-2
3 suppliers
inquiry
![Methyl 2-((1r,4r)-4-(4-(4-aMino-5-oxo-7,8-dihydropyriMido[5,4-f][1,4]oxazepin-6(5H)-yl)phenyl)cyclohexyl)acetate](/CAS/GIF/1109277-49-7.gif)
1109277-49-7
3 suppliers
inquiry
Yield:-
Reaction Conditions:
with triethylamine in acetonitrile at 80; for 6 h;
Steps:
1.4
Step 4. The title compound of Preparation 1 was prepared as follows. A slurry of methyl (trans-4-{4-[[4,6-dichloropyrimidin-5-yl)carbonyl](2-hydroxy-ethyl)amino]phenyl}cyclohexyl)acetate (4.78 g, 10.2 mmol, unpurified material from Step 3) and TEA (4.15 g, 41 mmol) in acetonitrile was stirred at 80° C. for 6 hours. The reaction was cooled, concentrated in vacuo, diluted into EtOAc, washed with water (3*), saturated aqueous brine, dried over sodium sulfate and concentrated in vacuo to afford a yellow solid. This material was slurried in methanol (10 mL), filtered, the solids washed with methanol (2*3 mL) and dried in vacuo to afford the title compound as a yellow solid, 4.03 g. 1H NMR (400 MHz, CDCl3): δ 8.75 (s, 1H), 7.22 (s, 4H), 4.75 (m, 2H), 4.03 (m, 2H), 3.63 (s, 3H), 2.50 (m, 1H), 2.23 (m, 2H), 1.87 (m, 5H), 1.44 (m, 2H), 1.19 (m, 2H). m/z=430.3 (M+1).
References:
US2009/36425,2009,A1 Location in patent:Page/Page column 13