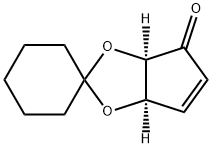
(1R,2R)-1,2-Dihydroxy-3-cyclopropen-5-one 1,2-Cyclohexyl Ketal synthesis
- Product Name:(1R,2R)-1,2-Dihydroxy-3-cyclopropen-5-one 1,2-Cyclohexyl Ketal
- CAS Number:111005-65-3
- Molecular formula:C11H14O3
- Molecular Weight:194.23
![(3aS,4S,6aR)-spiro[4,6a-dihydro-3aH-cyclopenta[d][1,3]dioxole-2,1'-cyclohexane]-4-ol](/CAS/20180601/GIF/111005-67-5.gif)
111005-67-5
0 suppliers
inquiry

111005-65-3
10 suppliers
$140.00/25mg
Yield:111005-65-3 821 mg
Reaction Conditions:
with sodium hydrogencarbonate;Dess-Martin periodane in dichloromethane; for 2 h;
Steps:
3 Compound 10
A solution of compound 9 (1.1 g, 4.91 mmol) in CH2C12 (100 mL) was degassed, followed by addition of 2 generation Grubbs’ catalyst (172 mg, 5 mmol%). The reaction mixture was stirred overnight at room temperature and concentrated under reduced pressure to give a brown dark residue, which was dissolved in CH2C12 (20 mL). Dess-Martin periodinane (DMP, 3.11 g, 7.34 mmol) and NaHCO3 (619 mg, 7.34 mmol) were added into the solution. After 2 h, the reaction was quenched by adding saturated Na2S2O3 (30 mL) and ether (100 mL) and stirred for 30 mm. The separate organic layer was dried over Na2SO4, concentrated and the residue purified with column chromatography (silica gel, EtOAc/Hexanes 1:8) to give compound 10 as a colorless oil (821 mg,86%). ‘H NMR (400 MHz, CDC13): 6 7.60 (dcl, J = 2.4, 3.6 Hz, 1 H), 6.20 (d, J = 5.2 Hz, 1 H), 5.27-5.24 (m, 1 H), 4.45 (d, J= 5.2 Hz, 1 H), 1.67-1.37 (m, 10 H).
References:
WO2015/13256,2015,A1 Location in patent:Paragraph 0060; 0061; 00106

123168-27-4
4 suppliers
inquiry

111005-65-3
10 suppliers
$140.00/25mg

108-94-1
527 suppliers
$12.00/50g

111005-65-3
10 suppliers
$140.00/25mg
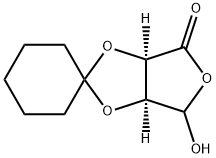
186803-48-5
8 suppliers
$475.00/500mg

111005-65-3
10 suppliers
$140.00/25mg
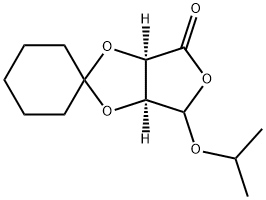
186790-80-7
7 suppliers
$140.00/50mg
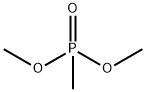
756-79-6
7 suppliers
$15.00/10g

111005-65-3
10 suppliers
$140.00/25mg