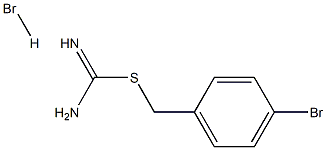
{[(4-bromophenyl)methyl]sulfanyl}methanimidamide hydrobromide synthesis
- Product Name:{[(4-bromophenyl)methyl]sulfanyl}methanimidamide hydrobromide
- CAS Number:111039-41-9
- Molecular formula:C8H10Br2N2S
- Molecular Weight:326.0514
Yield:111039-41-9 97%
Reaction Conditions:
in ethanol; for 2 h;Reflux;
Steps:
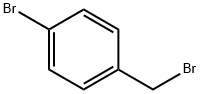
7 4-Bromobenzyl Carbamimidothioate Hydrobromide
1-Bromo-4-(bromomethyl)benzene (507 mg, 2.03 mmol) and 7 thiourea (129 mg, 1.69 mmol) were dissolved in 8 ethanol (20 mL). The reaction mixture was refluxed for 2 hours. The reaction mixture was cooled, and ethanol was removed under reduced pressure. The resulting residue was suspended in dichloromethane. The suspension was filtered to give a product as a white solid (535 mg, 97%); 1H NMR (300 MHz) (DMSO-d6) δ 9.21 (s, 2H), 9.01 (s, 2H), 7.59-7.55 (m, 2H), 7.40-7.36 (m, 2H), 4.48 (s, 2H); LC/MS RT=2.406 (M+H+: 246).
References:
US2019/31624,2019,A1 Location in patent:Paragraph 0417
873-75-6
308 suppliers
$6.00/5g
![{[(4-bromophenyl)methyl]sulfanyl}methanimidamide hydrobromide](/CAS/20180601/GIF/111039-41-9.gif)
111039-41-9
8 suppliers
$75.00/25mg