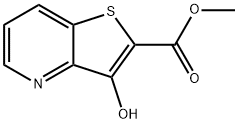
methyl 3-hydroxythieno[3,2-b]pyridine-2-carboxylate synthesis
- Product Name:methyl 3-hydroxythieno[3,2-b]pyridine-2-carboxylate
- CAS Number:111042-98-9
- Molecular formula:C9H7NO3S
- Molecular Weight:209.22

53636-56-9
142 suppliers
$10.00/1g

2365-48-2
357 suppliers
$14.00/25g
![methyl 3-hydroxythieno[3,2-b]pyridine-2-carboxylate](/CAS/20180703/GIF/111042-98-9.gif)
111042-98-9
4 suppliers
inquiry
Yield:111042-98-9 35%
Reaction Conditions:
Stage #1: methyl 3-bromopicolinate;Methyl thioglycolatewith potassium carbonate in acetonitrile;Heating / reflux;
Stage #2: with acetic acid in water at 20; pH=4;
Steps:
3.d EXAMPLE 3; 3-Methoxythieno[2,3-b]pyridine-2-carboxylic acid (8-benzyl-8-azabicyclo[3.2.1]oct-3β-yl)amide hydrochloride; d) 3-Hydroxythieno[3,2-b]pyridine-2-carboxylic acid methyl ester
This compound is described in J. Heterocycl. Chem. 1987, 24, 85. A mixture of 2.48 g of the product obtained in the step in example 3c) above (11.0 mmol, 1 eq.) with 1.03 ml of methyl thioglycolate (11.0 mmol, 1 eq.) in 100 ml of acetonitrile is reflux heated in the presence of 2.4 g of potassium carbonate (17.0 mmol, 1.5 eq.). The mixture is then allowed to return to ambient temperature, the solvent is vacuum evaporated and the residue taken up with water. Acetic acid (pH 4) is added and the precipitate formed is filtered. 852 mg of product (35%) is obtained. FP=184° C. NMR (1H, CDCl3): 4.00 (s; 3H), 7.42 (dd, J=8.2, 4.4 Hz; 1H), 8.11 (dd, J=8.2, 1.3 Hz; 1H), 8.79 (dd, J=4.4, 1.3 Hz; 1H), 9.96 (large; 1H).
References:
US2005/80085,2005,A1 Location in patent:Page/Page column 6