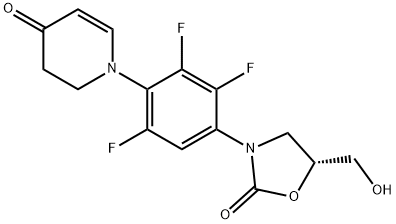
(R)-5-(hydroxymethyl)-3-(2,3,5-trifluoro-4-(4-oxo-3,4-dihydropyridin-1(2H)-yl)phenyl)oxazolidin-2-one synthesis
- Product Name:(R)-5-(hydroxymethyl)-3-(2,3,5-trifluoro-4-(4-oxo-3,4-dihydropyridin-1(2H)-yl)phenyl)oxazolidin-2-one
- CAS Number:1112968-16-7
- Molecular formula:C15H13F3N2O4
- Molecular Weight:342.27

1239515-40-2
9 suppliers
inquiry
60456-26-0
300 suppliers
$14.14/1gm:

1112968-16-7
1 suppliers
inquiry
Yield:1112968-16-7 65%
Reaction Conditions:
Stage #1: C16H17F3N2O3with lithium tert-butoxide in tetrahydrofuran;acetonitrile at 5;Inert atmosphere;
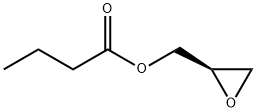
Stage #2: (R)-glycidyl butyrate in tetrahydrofuran;acetonitrile at 17 - 20;
Steps:
1
Compound of Example 1Intermediate 5 (17.0 g, 49 mmol) was dissolved in anhydrous THF (10 mL) and CH3CN (34 mL) at 5° C. under nitrogen. ButOLi (powder, 4.3 g, 54 mmol) was added slowly and the resulting solution was stirred for another 1 h at 5° C. (R)-Glycidyl butyrate (14.1 mL, 99 mmol) was added dropwise. The mixture was stirred for 3 h and allowed to warm up to 17-20° C. The reaction mixture was cooled to 5° C. and quenched carefully with aq. acetic acid solution (1.0 M, 85 mL). The solid was dissolved after stirring for 3 h at 5° C. The mixture was condensed under vacuum, and the residue was re-dissolved in DCM (100 mL) and washed with water (50 mL). The DCM phase was condensed under vacuum. The residue was dissolved in CH3OH (34 mL) and cooled to 10° C. Then 10% aq. K2CO3 solution (30 mL) was added slowly. The mixture was warmed up to 20° C. and stirred for 2 h. After cooling to 5° C., aq. acetic acid solution (1.0 M, 50 mL) was added dropwise to adjust the pH to ca. 6-7. The mixture was extracted with DCM (3×60 mL). The combined DCM layers were washed with brine (80 mL) and 1% aq. HCl (30 mL), dried over MgSO4, filtered and condensed on vacuum. The solid obtained was washed with a mixture of EtOAc (15 mL) and heptane (15 mL) for 2 h at 75° C., filtered and dried. The product was obtained as an off-white solid (11.1 g, 65%). 1H NMR (400 MHz, DMSO-d6): 7.55 (m, 1H); 7.46 (d, J=7.6 Hz, 1H); 5.24 (t, J=4.8 Hz, 1H); 5.04 (d, J=8.0 Hz, 1H); 4.75 (m, 1H); 4.09 (t, J=4.8 Hz, 1H); 3.86 (t, J=7.2 Hz, 3H); 3.67 (m, 1H); 3.55 (m, 1H); 3.30 (s, 2H). MS (m/z): 343 [M+H].
References:
US2010/204477,2010,A1 Location in patent:Page/Page column 16

1112968-96-3
2 suppliers
inquiry

60456-26-0
300 suppliers
$14.14/1gm:

1112968-16-7
1 suppliers
inquiry

1112968-95-2
4 suppliers
inquiry

1112968-16-7
1 suppliers
inquiry

1112968-93-0
1 suppliers
inquiry

1112968-16-7
1 suppliers
inquiry

1112968-91-8
3 suppliers
inquiry

1112968-16-7
1 suppliers
inquiry