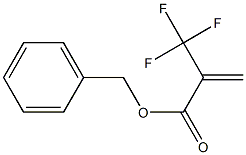
2-Propenoic acid, 2-(trifluoromethyl)-, phenylmethyl ester synthesis
- Product Name:2-Propenoic acid, 2-(trifluoromethyl)-, phenylmethyl ester
- CAS Number:111339-17-4
- Molecular formula:C11H9F3O2
- Molecular Weight:230.1832
Yield:111339-17-4 82%
Reaction Conditions:
with triethylamine in diethyl ether at -40 - 0; for 2 h;
Steps:
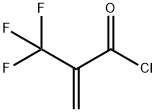
1 Preparation of α,α,α-trifluoromethacrylic acid benzyl ester
REFERENCE EXAMPLE 1; Preparation of α,α,α-trifluoromethacrylic acid benzyl ester. Into a 1,000 ml three-necked round-bottomed flask equipped with a dropping funnel and a stirrer, benzyl alcohol (54.6 g, 0.51 mol) and diethyl ether (600 ml) were charged, and then, the α,α,α-trifluoromethacrylic acid chloride (80.0 g, 0.51 mol) obtained as described above, was dropwise added at -40°C, and then a mixture comprising triethylamine (56.2 g, 0.56 mol) and diethyl ether (100 ml) was dropwise added. The mixture was stirred at -40°C for one hour and then further stirred at 0°C for one hour. To the reaction solution, a saturated ammonium chloride aqueous solution was added, and then, extracted with diethyl ether and dried over anhydrous magnesium sulfate, and the solvent was distilled off under reduced pressure. The residue was purified by silica gel column chromatography (hexane/ethyl acetate=9/1:vol/vol) to obtain α,α,α-trifluoromethacrylic acid benzyl ester (95.3 g) (yield: 82%). Analytical results 1H-NMR(200 MHz,CDCl3) δ 7.37-7.29(m, 5H), 6.70-6.67(m, 1H), 6.38-6.37(m, 1H), 5.25(s, 2H).
References:
EP1422226,2004,A1 Location in patent:Page 10