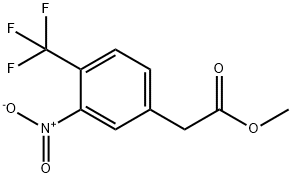
METHYL 2-(3-NITRO-4-(TRIFLUOROMETHYL)PHENYL)ACETATE synthesis
- Product Name:METHYL 2-(3-NITRO-4-(TRIFLUOROMETHYL)PHENYL)ACETATE
- CAS Number:1116624-06-6
- Molecular formula:C10H8F3NO4
- Molecular Weight:263.17

67-56-1
757 suppliers
$9.00/25ml
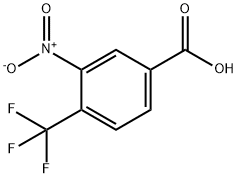
116965-16-3
123 suppliers
$7.00/250mg
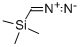
18107-18-1
211 suppliers
$33.00/5g

1116624-06-6
5 suppliers
inquiry
Yield:1116624-06-6 49%
Reaction Conditions:
Stage #1: 3-nitro-4-(trifluoromethyl)benzoic acidwith thionyl chloride for 6 h;Reflux;
Stage #2: diazomethyl-trimethyl-silanewith triethylamine in tetrahydrofuran;hexane at 0; for 16 h;Arndt-Eistert Homologation;
Stage #3: methanolwith silver(I) acetate;triethylamine at 50; for 4 h;
Steps:
(0150) Methyl 2-(3-nitro-4-(trifluoromethyl)phenyl)acetate (IV-28) :
A solution of No.87 3-nitro-4-(trifluoromethyl)benzoic acid IV-23 (1.4 g, 5.95 mmol) in No.88 thionyl chloride (10 mL) was heated to reflux with stirring for 6 h. The yellow solution was concentrated to give an oil, which was diluted in anhydrous No.37 THF (10 mL) and cooled to 0°C. with stirring. This solution was added dropwise to a stirred solution of No.89 triethylamine (1.91 mL, 13.7 mmol) and TMSCHN2 (2 M in hexanes, 3.6 mL, 7.2 mmol) in THF at 0°C. The mixture was stirred at 0°C. for 16 h, filtered with the aid of additional ether, then concentrated to give an orange oil that was diluted in No.67 MeOH (20 mL). To this solution was added triethylamine (913 μL, 6.55 mmol) followed by No.90 silver (I) acetate (596 mg, 3.57 mmol) then heated at 50°C. for 4 h before it was concentrated. The crude residue was diluted in No.33 ethyl acetate and filtered through a Celite pad. The filtrate was washed successively with saturated sodium bicarbonate (230 mL), water (30 mL), saturated ammonium chloride (230 mL), and brine (30 mL), then dried (Na2SO4), concentrated under reduced pressure, and chromatographed (ethyl acetate/hexanes, 1:9) to yield the desired No.91 product as an orange oil (764 mg, 49%). 1H NMR (500 MHz, CDCl3 δ 7.83 (s, 1H), 7.78 (d, J=8.1Hz, 1H), 7.65 (d, J=7.9 Hz, 1H), 3.77 (s, 2H), 3.73 (s, 4H). 13C NMR (126 MHz, CDCl3) δ 170.07 (s), 148.17 (s), 140.26 (s), 133.70 (s), 128.22 (q, J=5.2 Hz), 122.56 (q, J=34.3 Hz), 122.04 (q, J=273.2 Hz), 52.69 (s), 40.19 (s). 19F NMR (376 MHz, CDCl3) δ -60.41 (3F, s). HRMS [M-F]+ Calcd for C10H8F3NO4 244.0416; found 244.0410.
References:
US2018/98952,2018,A1 Location in patent:Paragraph 0141; 0150

67-56-1
757 suppliers
$9.00/25ml
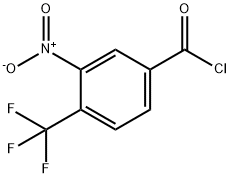
1049103-00-5
3 suppliers
inquiry
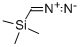
18107-18-1
211 suppliers
$33.00/5g

1116624-06-6
5 suppliers
inquiry