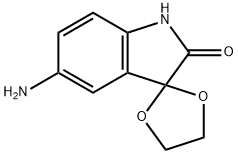
5'-AMINOSPIRO[1,3-DIOXOLANE-2,3'-INDOL]-2'(1'H)-ONE synthesis
- Product Name:5'-AMINOSPIRO[1,3-DIOXOLANE-2,3'-INDOL]-2'(1'H)-ONE
- CAS Number:113207-59-3
- Molecular formula:C10H10N2O3
- Molecular Weight:206.2
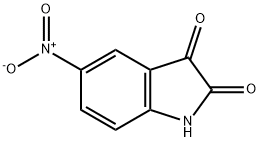
![5'-nitro-1',2'-dihydrospiro[1,3-dioxolane-2,3'-indole]-2'-one](/CAS/20180713/GIF/75822-55-8.gif)
75822-55-8
7 suppliers
inquiry
![5'-AMINOSPIRO[1,3-DIOXOLANE-2,3'-INDOL]-2'(1'H)-ONE](/CAS/GIF/113207-59-3.gif)
113207-59-3
8 suppliers
$110.00/500mg
Yield:113207-59-3 96%
Reaction Conditions:
with 10% Pd/C;hydrogen in ethyl acetate at 20; under 2250.23 - 7500.75 Torr;Flow reactor;
Steps:
4.1.2. Catalytic nitro-group reduction (2). 4.1.2.1. Method A
General procedure: The solution of nitro-spiro[1,3-dioxolan-2,3′-indol]-2′(1′H)-one in ethyl acetate (2 mg/mL) was hydrogenated using continuous flow reactor H-Cube (ThalesNano Nanotechnology Inc., Hungary) under the following conditions: Pd/C (10 %), 3-10 bar H2, 1,0-2,0 mL/min, rt. After the reaction proceeded the solvent was removed in vacuum.
References:
Zaryanova, Ekaterina V.;Ignatov, Alexander A.;Lozynskaya, Nataly A. [Tetrahedron,2017,vol. 73,# 49,p. 6887 - 6893]
611-09-6
188 suppliers
$6.00/1g
![5'-AMINOSPIRO[1,3-DIOXOLANE-2,3'-INDOL]-2'(1'H)-ONE](/CAS/GIF/113207-59-3.gif)
113207-59-3
8 suppliers
$110.00/500mg
91-56-5
437 suppliers
$5.00/25g
![5'-AMINOSPIRO[1,3-DIOXOLANE-2,3'-INDOL]-2'(1'H)-ONE](/CAS/GIF/113207-59-3.gif)
113207-59-3
8 suppliers
$110.00/500mg