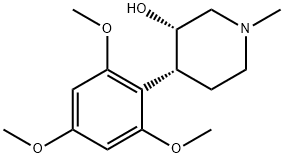
3-PIPERIDINOL, 1-METHYL-4-(2,4,6-TRIMETHOXYPHENYL)-, (3S,4R)- synthesis
- Product Name:3-PIPERIDINOL, 1-METHYL-4-(2,4,6-TRIMETHOXYPHENYL)-, (3S,4R)-
- CAS Number:113225-19-7
- Molecular formula:C15H23NO4
- Molecular Weight:281.35
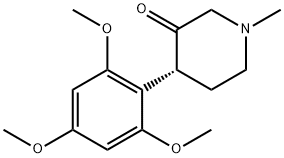
205506-14-5
4 suppliers
inquiry

113225-19-7
11 suppliers
inquiry
Yield:113225-19-7 88.6%
Reaction Conditions:
with lithium tri-tert-butylborohydride in tetrahydrofuran at 0; for 2 h;Inert atmosphere;Large scale;
Steps:
4 Example 4: Manufacturing Method of cis-(-)-flocinopiperidol
Under a nitrogen atmosphere, a THF solution (1210.6 g) of lithium triisobutylborohydride was cooled to 0° C. The solution obtained in Example 3 (4461.0 g) was added dropwise at an internal temperature of 0° C. The mixture was incubated and stirred for 2 hours at 0° C. The solution was added dropwise at 10° C. or lower to an aqueous 15% potassium hydroxide solution (1875 g) cooled to 5° C. After the addition, the mixture was warmed to 15° C., and 30% hydrogen peroxide water (426.7 g) was added dropwise at an internal temperature of 30° C. or lower. After stirring for 30 minutes at 20° C., an aqueous 24% sodium hydrogen sulfite solution (413.55 g) was added dropwise at 20° C., and the mixture was stirred for 1 hour. The mixture was checked for peroxides with potassium iodide-starch paper, and toluene (1752 g) was added. After 10 minutes of stirring, the aqueous layer was removed, and the organic layer (7403 g) was concentrated under reduced pressure to 2802 g. Toluene (1752 g) was added again, and concentrated under reduced pressure to 958 g. Toluene (1456 g) was added. An aqueous 5% potassium dihydrogen phosphate solution (525.7 g) was added at 20° C. The pH was adjusted to 6.37 with concentrated hydrochloric acid (56 g). A portion of the post-separation bottom layer (37.2 g) was added dropwise at 20° C. to an aqueous 7% potassium hydroxide solution (876 g), and cis-(-)-flocinopiperidol (0.88 g) was added. The remainder of the post-separation bottom layer (710.1 g) was added dropwise at 20° C. The mixture was incubated and stirred for 2 hours at 20° C. The precipitate was filtered out, washed twice with water (200 g) and then dried to obtain cis-(-)-flocinopiperidol (156.4 g, yield: 88.6%, HPLC purity: 99.91 area %, optical purity: 99.3% ee).1H-NMR (400 MHz, CDCl3) δ: 1.37-1.42 (m, 1H), 1.65 (s, 3H), 2.04 (ddd, J=11.6, 11.6, 2.7 Hz, 1H), 2.12-2.15 (m, 1H), 2.31 (s, 3H), 2.83-2.94 (m, 1H), 2.96-3.02 (m, 2H), 3.34-3.39 (m, 1H), 3.81 (s, 9H), 3.84-3.85 (m, 1H), 6.17 (s, 2H)
References:
US2022/220072,2022,A1 Location in patent:Paragraph 0128-0130
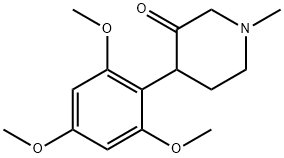
113225-10-8
24 suppliers
$36.00/250mg

113225-19-7
11 suppliers
inquiry