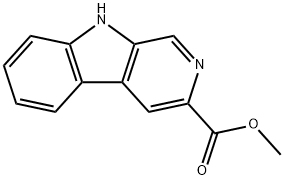
methyl 1-formyl-9H-pyrido[3,4-b]indole-3-carboxylate synthesis
- Product Name:methyl 1-formyl-9H-pyrido[3,4-b]indole-3-carboxylate
- CAS Number:113247-36-2
- Molecular formula:C14H10N2O3
- Molecular Weight:254.24
Yield:113247-36-2 45%
Reaction Conditions:
with ferrous(II) sulfate heptahydrate;sulfuric acid;dihydrogen peroxide at 10 - 15;
Steps:
4.2.5. Synthesis of methyl 1-substituted-9H-pyrido[3,4-b]indole-3-carboxylate (4m-4p)
General procedure: To a solution of β-carboline derivative 4a (0.34 g, 1.5 mmol),50 mL solvent (formamide for 4m, formaldehyde for 4n, methanolfor 4o, N-methylformamide for 4p) and 1 mL concentrated sulfuricacid were placed. The mixture was stirred at 10-15 °C. A saturatedsolution of FeSO4*7H2O and H2O2 (30% solution in water) wereadded simultaneously over a period of 10 min, and stirringcontinued. The addition of FeSO4*7H2O and H2O2 can be repeateduntil there was no starting material left (TLC control). The reactionmixture was poured into water (150 mL), followed by neutralizedwith saturated solution of sodium carbonate and extracted withethyl acetate (3 x 100 mL). The combined organic layers werewashed with brine (1 x 100 mL) and dried over anhydrous Na2SO4,and the solvent was removed under vacuum. The residue was purifiedby flash column chromatography with ethyl acetate and petroleumether (v/v 1:5*1:1) as eluent to afford 4m-4p.
References:
Sheng, Tao;Kong, Mengmeng;Wang, Yujie;Wu, HuiJun;Gu, Qin;Chuang, Anita Shyying;Li, Shengkun;Gao, Xuewen [European Journal of Medicinal Chemistry,2021,vol. 222,art. no. 113563]
![9H-Pyrido[3,4-b]indole-3-carboxylic acid, 1-(dimethoxymethyl)-, methyl ester](/CAS/20200515/GIF/129609-50-3.gif)
129609-50-3
0 suppliers
inquiry
![methyl 1-formyl-9H-pyrido[3,4-b]indole-3-carboxylate](/CAS/20180703/GIF/113247-36-2.gif)
113247-36-2
18 suppliers
inquiry
16641-82-0
17 suppliers
inquiry
![methyl 1-formyl-9H-pyrido[3,4-b]indole-3-carboxylate](/CAS/20180703/GIF/113247-36-2.gif)
113247-36-2
18 suppliers
inquiry
4299-70-1
36 suppliers
$337.00/250mg
![methyl 1-formyl-9H-pyrido[3,4-b]indole-3-carboxylate](/CAS/20180703/GIF/113247-36-2.gif)
113247-36-2
18 suppliers
inquiry
73-22-3
947 suppliers
$5.00/250mg
![methyl 1-formyl-9H-pyrido[3,4-b]indole-3-carboxylate](/CAS/20180703/GIF/113247-36-2.gif)
113247-36-2
18 suppliers
inquiry