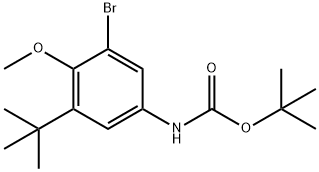
tert-Butyl (3-bromo-5-(tert-butyl)-4-methoxyphenyl)carbamate synthesis
- Product Name:tert-Butyl (3-bromo-5-(tert-butyl)-4-methoxyphenyl)carbamate
- CAS Number:1132940-57-8
- Molecular formula:C16H24BrNO3
- Molecular Weight:358.27
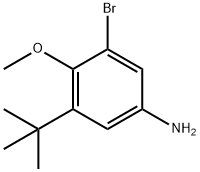
1132940-59-0
8 suppliers
inquiry

24424-99-5
824 suppliers
$13.50/25G

1132940-57-8
7 suppliers
inquiry
Yield:1132940-57-8 75%
Reaction Conditions:
in tetrahydrofuran; for 2 h;Heating / reflux;
Steps:
1.H
[00502] Part H. Preparation of tert-butyl 3-bromo-5-tert-butyl-4-methoxyphenylcarbamate.; [00503] A solution ofthe product from Part G (960mg, 3.33mmol) in methanol (17mL) was treated with5 % platinum on sulfided carbon (lOOmg), followed by hydrogenation under balloon pressure for 3h, and then filtered through celite and concentrated under vacuum to afford the 3-bromo-5-tert-butyl-4- methoxyaniline as a yellow oil (860mg, 3.33mmol, 100%). A solution of this material in THF (17mL) was treated with di-tert-butyl dicarbonate (800mg, 3.66mmol) followed by warming at reflux for 2h.Concentration under vacuum afforded a beige solid, which was purified by silica gel column chromatography eluting with EtOAc/hexanes. Solid was triturated with hexanes, collected by filtration, and dried under vacuum to give the title compound as a nearly white solid (890mg, 75 %).
References:
WO2009/39134,2009,A1 Location in patent:Page/Page column 105