
5-((6-(Methyl(tetrahydro-2H-pyran-4-yl)amino)pyrimidin-4-yl)amino)pyrazine-2-carbonitrile synthesis
- Product Name:5-((6-(Methyl(tetrahydro-2H-pyran-4-yl)amino)pyrimidin-4-yl)amino)pyrazine-2-carbonitrile
- CAS Number:1137475-38-7
- Molecular formula:C15H17N7O
- Molecular Weight:311.34
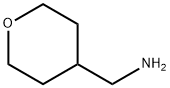
130290-79-8
293 suppliers
$6.00/1g

1137475-05-8
3 suppliers
inquiry

1137475-38-7
4 suppliers
inquiry
Yield:1137475-38-7 41%
Reaction Conditions:
with triethylamine in acetonitrile at 145; for 0.5 h;Microwave irradiation;
Steps:
33
A solution of 5-(6-chloropyrimidin-4-ylamino)pyrazine-2-carbonitrile (20 mg, 0.086 mmol), 4-(aminomethyl)tetrahydropyran (18 mg, 0.17 mmol), and triethylamine (0.02 mL, 0.13 mmol) in MeCN (0.2 mL ) was heated to 145°C for 30 minutes by microwave irradiation. The mixture was cooled and solvent was removed by evaporation. The crude material was redissolved in a mixture of dichloromethane (89%), MeOH (10%), 0.88 s.g. NH3 (1%) and adsorbed onto a solvent-conditioned Trikonex silica chromatography column. Elution with the same solvent mixture gave 5-(6-((tetrahydro-2H-pyran-4-yl)methylamino) pyrimidin-4-ylamino)pyrazine-2-carbonitrile as a yellow powder (11 mg, 41%).1H NMR (500 MHz, DMSO) δ 1.15-1.24 (2H, m), 1.60 (2H1 d, J = 12.5 Hz), 1.73-1.83 (1 H, m), 3.15-3.28 (4H, m), 3.84 (2H, dd, J = 3.0, 11.0 Hz), 7.00 (1 H, br s), 7.50 (1H, br s), 8.22 (1 H, s), 8.75 (1 H, s), 8.86 (1 H, br s), 10.59 (1 H, br s). LCMS (3) Rt 2.79 min; m/z (ESI+) 312 (MH+).
References:
WO2009/44162,2009,A1 Location in patent:Page/Page column 141
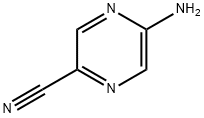
113305-94-5
173 suppliers
inquiry

1137475-38-7
4 suppliers
inquiry