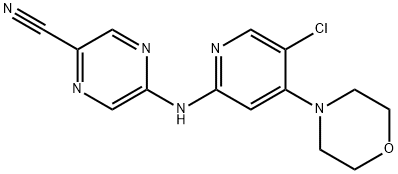
5-((5-Chloro-4-morpholinopyridin-2-yl)amino)pyrazine-2-carbonitrile synthesis
- Product Name:5-((5-Chloro-4-morpholinopyridin-2-yl)amino)pyrazine-2-carbonitrile
- CAS Number:1137476-13-1
- Molecular formula:C14H13ClN6O
- Molecular Weight:316.75
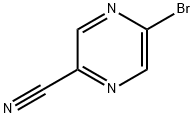
221295-04-1
107 suppliers
$31.00/250mg
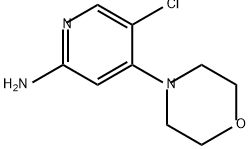
1137476-11-9
0 suppliers
inquiry

1137476-13-1
4 suppliers
inquiry
Yield:1137476-13-1 20%
Reaction Conditions:
Stage #1: 5-bromopyrazine-2-carbonitrile;5-chloro-4-morpholinopyridin-2-aminewith sodium t-butanolate;palladium diacetate;2,2'-bis-(diphenylphosphino)-1,1'-binaphthyl in N,N-dimethyl-formamide;toluene at 140; for 0.333333 h;Microwave irradiation;
Stage #2: with ammonia in methanol;N,N-dimethyl-formamide;toluene;
Steps:
78-B
5-Chloro-4-morpholinopyridin-2-amine (68 mg, 0.31 mmol), 4-bromo-cyanopyrazine (30 mg, 0.20 mmol), sodium te/f-butoxide (45 mg, 0.47 mmol), Pd(OAc)2 (3 mg, 0.01 mmol) and BINAP (0.030 g, 0.05 mmol) were mixed under argon atmosphere before addition of mixture of DMF in toluene (2:1 , 0.7 mL). The reaction mixture was heated to 1400C by microwave irradiation for 20 minutes. The reaction mixture was purified by ion exchange chromatography on SCX-II acidic resin (500 mg) eluting with methanol, then 2M ammonia-methanol. The basic fractions were combined and solvent was evaporated. Flash chromoatography on silica, eluting with ethyl acetate - hexane (1:1) gave 5-(5- chloro-4-morpholinopyridin-2-ylamino)pyrazine-2-carbonitrile as a yellow solid (20 mg, 20%).1H NMR (500 MHz, (CD3)2CO) δ 3.14-3.16 (4H, m), 3.76-3.79 (4H, m), 7.52 (1H, s), 8.22 (1H, s), 8.77 (1 H, s), 9.02 (1H1 s), 10.77 (1H, s). LCMS (3) Rt 4.55 min; m/z (ESI+) 317 (MH+).
References:
WO2009/44162,2009,A1 Location in patent:Page/Page column 168
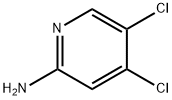
188577-68-6
126 suppliers
$17.00/100mg

1137476-13-1
4 suppliers
inquiry