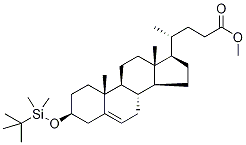
(3β)-3-(tert-ButyldiMethylsilyl)oxy-chol-5-en-24-oic Acid Methyl Ester synthesis
- Product Name:(3β)-3-(tert-ButyldiMethylsilyl)oxy-chol-5-en-24-oic Acid Methyl Ester
- CAS Number:114011-35-7
- Molecular formula:C31H54O3Si
- Molecular Weight:502.84

67-56-1
737 suppliers
$9.00/25ml
5255-17-4
83 suppliers
$60.00/10mg

18162-48-6
668 suppliers
$9.00/5g

114011-35-7
5 suppliers
$145.00/25mg
Yield:114011-35-7 99%
Reaction Conditions:
Stage #1: methanol;3β-hydroxy-5-cholenoic acidwith toluene-4-sulfonic acid at 60;
Stage #2: tert-butyldimethylsilyl chloridewith 1H-imidazole;dmap in tetrahydrofuran;N,N-dimethyl-formamide at 20; for 1 h;
Steps:
1
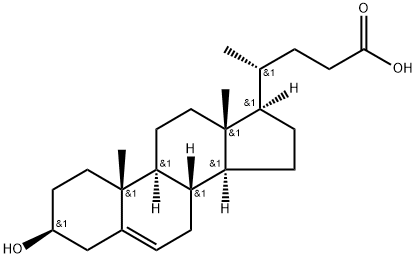
95mg p-TsOH (p-TsOH) was added to a solution of 2.0g 3-hydroxy-5-cholenoic acid in 30mL methanol,Heat to 60 ° C and stir for 2 hours.After the reaction is completed, the solvent is concentrated and dissolved in 100 mL of ethyl acetate.Successively washed with saturated aqueous sodium bicarbonate solution and saturated brine,Then dried over anhydrous sodium sulfate, filtered to remove sodium sulfate,After concentration, the crude product was dissolved in a mixed solvent of 3mL DMF and 5mL THF,Add 680 mg imidazole, 64 mg DMAP and 1.5 g TBSCl, and stir at room temperature for 1 hour.After the reaction was completed, 20 mL of saturated sodium bicarbonate solution was added to quench the reaction, and the organic phase was separated.The aqueous phase was extracted three times with dichloromethane and incorporated into the organic phase,Successively washed with saturated aqueous sodium bicarbonate solution and saturated brine,It was then dried over anhydrous sodium sulfate, filtered to remove sodium sulfate, and concentrated to obtain a crude product.The crude product was isolated by column chromatography (200-300 mesh silica gel, eluent was n-hexane: ethyl acetate = 20: 1) to obtain pure intermediate 18.
References:
CN110894209,2020,A Location in patent:Paragraph 0085; 0140; 0142-0143; 0151
20231-57-6
27 suppliers
$225.00/100MG

18162-48-6
668 suppliers
$9.00/5g

114011-35-7
5 suppliers
$145.00/25mg
![Chola-5,22-dien-24-oic acid, 3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, methyl ester, (3β,22E)- (9CI)](/CAS/20210305/GIF/164298-05-9.gif)
164298-05-9
0 suppliers
inquiry

114011-35-7
5 suppliers
$145.00/25mg

5255-17-4
83 suppliers
$60.00/10mg

114011-35-7
5 suppliers
$145.00/25mg
![Pregn-5-ene-20-methanol, 3-[[(1,1-dimethylethyl)dimethylsilyl]oxy]-, (3β,20S)-](/CAS/20210305/GIF/69454-88-2.gif)
69454-88-2
0 suppliers
inquiry

114011-35-7
5 suppliers
$145.00/25mg