
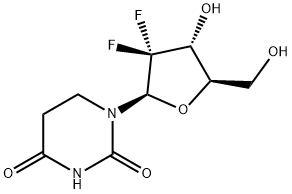
(R)-1-((2R, 4R, SR)-3,3-difluoro-4-hydroxy (hydroxymethyl) tetrahydrofuran-2-yl)-4-hydroxytetrahydropyrimidin-2(1H)-one synthesis
- Product Name:(R)-1-((2R, 4R, SR)-3,3-difluoro-4-hydroxy (hydroxymethyl) tetrahydrofuran-2-yl)-4-hydroxytetrahydropyrimidin-2(1H)-one
- CAS Number:1141397-80-9
- Molecular formula:C9H14F2N2O5
- Molecular Weight:268.21

1141397-48-9
1 suppliers
inquiry

1141397-80-9
32 suppliers
inquiry
Yield:97.2 % de
Reaction Conditions:
Stage #1: 2’-deoxy-2’,2’-difluorotetrahydrouridinewith 1,8-diazabicyclo[5.4.0]undec-7-ene in water;acetonitrile at 25; for 74 h;Large scale;
Stage #2: in water;acetonitrile at 0 - 40; for 16 h;Large scale;Reagent/catalyst;
Steps:
5 Example 5 Preparation of compound 1 from compound 2 using DBU
An additional batch of compound I was prepared from compound 2 using DBU. Compound 2 (5.69 kg based on theoretical calculations of material prepared substantially as described in a scaled-up version of the examples above, 21.2 mol, containing 1.01 L of water by F analysis) was suspended in a mixture of acetonitrile (19.4 L) and water (1.51 L). The mixture was stirred for 10 min to give a suspension of fine powder and treated with 1,8- diazabicyclo[5.4.0]undec-7~ene (150 g, 1.06 mol, 0.05 equiv). Stirring was continued at 25 °C for 2 h. A sample of the supernatant of the reaction mixture was submitted for HPLC to check if the supernatant solution was 50/50 compound 1/compound 6. The supernatant was a 50/50 mixture of compound 1/compound 6. No additional 1,8-diazabicyclo[5.4.0]undec-7- ene (150 g, 1.06 mol, 0.05 equiv was needed. The mixture was then stirred at 25 °C for 3 days. The resulting precipitate was filtered, and washed with a 1:7 (v/v) mixture of water and acetonitrile (4.3 kg) and then twice with acetonitrile (4.27 kg each). The filter cake was dried over nitrogen purge to afford compound 1 (3.53 kg, 59% yield; compound 1/compound 6 = 95/5). [0078] A combined portion of compound 1 from the batch above and a second batch prepared in substantially the same manner (5.46 kg, 20.4 mol) was treated with acetonitrile (17.5 L) and water (4.4 L), and heated to 40 °C for 4 h. The mixture was then cooled to 0 °C over 4 h and stirred at 0 °C for 12 h. The precipitate was filtered and washed with a 1:6 (v/v) mixture of water and acetonitrile (4.5 kg) and then twice with acetonitrile (4.3 kg each). The filter cake was dried over nitrogen purge to afford compound 1 (3.97 kg, 73% recovery; compound 1/compound 6 = 98.6/1.4).
References:
WO2015/66162,2015,A1 Location in patent:Paragraph 0077; 0078

95058-81-4
516 suppliers
$6.00/100mg

1141397-80-9
32 suppliers
inquiry
1141397-81-0
1 suppliers
inquiry
1141397-77-4
3 suppliers
inquiry

1141397-77-4
3 suppliers
inquiry

1141397-82-1
1 suppliers
inquiry

1141397-80-9
32 suppliers
inquiry
1141397-81-0
1 suppliers
inquiry

1141397-77-4
3 suppliers
inquiry

1141397-80-9
32 suppliers
inquiry