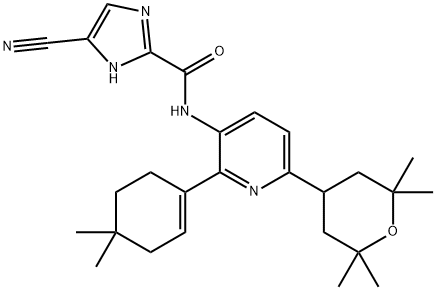
4-cyano-N-(2-(4,4-dimethylcyclohex-1-en-1-yl)-6-(2,2,6,6-tetramethyltetrahydro-2H-pyran-4-yl)pyridin-3-yl)- 1H-imidazole-2-carboxamide synthesis
- Product Name:4-cyano-N-(2-(4,4-dimethylcyclohex-1-en-1-yl)-6-(2,2,6,6-tetramethyltetrahydro-2H-pyran-4-yl)pyridin-3-yl)- 1H-imidazole-2-carboxamide
- CAS Number:1142363-52-7
- Molecular formula:C27H35N5O2
- Molecular Weight:461.6

1142363-66-3
2 suppliers
inquiry

1142363-52-7
55 suppliers
inquiry
Yield:1142363-52-7 75%
Reaction Conditions:
with tetrabutyl ammonium fluoride in N,N-dimethyl-formamide at 70;
Steps:
15.h
h) 4-Cyano-1H-imidazole-2-carboxylic acid[2-(4,4-dimethyl-cyclohex-1-enyl)-6-(2,2,6,6-tetramethyl-tetrahydro-pyran-4-yl)-pyridin-3-yl]-amide To a solution of 4-cyano-1-(2-trimethylsilanyl-ethoxymethyl)-1H-imidazole-2-carboxylic acid[2-(4,4-dimethyl-cyclohex-1-enyl)-6-(2,2,6,6-tetramethyl-tetrahydro-pyran-4-yl)-pyridin-3-yl]-amide (as prepared in the previous example, 17.6 g, 0.0290 mol) in DMF (30 mL) was added solid TBAF hydrate (16.6 g, 0.0630 mol). The resulting mixture was heated at 70° C. overnight. The reaction mixture was allowed to cool to RT and partitioned between EtOAc (200 mL) and water (200 mL). The organic layer was separated, the aqueous layer was washed with EtOAc (3*100 mL) and the organic layers were combined, dried (Na2SO4) and concentrated. The resulting residue was dried under high vacuum to remove residual DMF. The residue was purified on silica gel (0-50% EtOAc/hexane). The resulting solid was then suspended in 25% ether/hexane and sonicated for 10 min. The product was collected by suction filtration and dried in a vacuum oven at 60° C. for 12 h to obtain the title compound as a white solid (10.2 g, 75%.) 1H-NMR (DMSO; 400 MHz): δ 14.26 (s, 1H), 10.02 (s, 1H), 8.32 (s, 1H), 8.12 (d, 1H, J=8.3 Hz), 7.24 (d, 1H, J=8.3 Hz), 5.86 (br s, 1H), 3.23 (m, 1H), 2.40 (m, 2H), 1.91 (m, 2H), 1.74 (dd, 2H, J=12.9, 3.3 Hz), 1.48 (m, 4H), 1.30 (s, 6H), 1.15 (s, 6H), 0.96 (s, 6H). Mass spectrum (ESI, m/z): Calcd. for C27H35N5O2, 462.2 (M+H), found 462.3.
References:
US2009/105296,2009,A1 Location in patent:Page/Page column 45