
[1,1'-Biphenyl]-4-carboxylic acid, (3aR,4R,5R,6aS)-hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2-oxo-2H-cyclopenta[b]furan-5-yl ester synthesis
- Product Name:[1,1'-Biphenyl]-4-carboxylic acid, (3aR,4R,5R,6aS)-hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2-oxo-2H-cyclopenta[b]furan-5-yl ester
- CAS Number:114488-91-4
- Molecular formula:C31H27F3O6
- Molecular Weight:552.54
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-hexahydro-2-oxo-4-[(1E)-3-oxo-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2H-cyclopenta[b]furan-5-yl ester](/CAS2/GIF/54142-64-2.gif)
54142-64-2
55 suppliers
inquiry
![[1,1'-Biphenyl]-4-carboxylic acid, (3aR,4R,5R,6aS)-hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2-oxo-2H-cyclopenta[b]furan-5-yl ester](/CAS/20180702/GIF/114488-91-4.gif)
114488-91-4
16 suppliers
inquiry
Yield:114488-91-4 73.51%
Reaction Conditions:
with (3aR)-1-methyl-3,3-diphenyl-tetrahydro-pyrrolo[1,2-c][1,3,2]oxazaborole;benzo[1,3,2]dioxaborole in dichloromethane at -7 - 28; for 3 h;
Steps:
2.4 Step 4: Preparation of compound of formula III {(3aR, 4R, 5R, 6aS) -4 - ((R, E) -3- hydroxy- 4- (3- (trifluoromethyl) phenoxy) Enyl) -2-oxohexahydro-2H-cyclopenta [b] furan-5-ylbiphenyl-4- carboxylate}
Catecholborane (2.29 g, 19.072 mmol, 2.1 eq) was dissolved in 30 mL of methylene chloride. R-Me CBS (1M Tol) (6 mL, 5.994 mmol, 0.66 eq) was added dropwise at 28 deg C; 0 deg C for 5 min. Compound (II) (5 g, 9.082 mmol) prepared in Step 3 at -7 ° C was dissolved in 30 mL of methylene chloride and added dropwise for 1 hour and stirred for 2 hours. The organic layer was washed with 50 mL of water and 100 mL of a 10% NaCl solution. The organic layer was further washed with brine (100 mL), and the organic layer was dried over anhydrous, and then the solvent was concentrated under reduced pressure. The yellowish oil was collected by column chromatography with ethyl acetate: hexane = 1: 1 to give a yellow oil (3.69 g, 73.51%).
References:
KR2017/25682,2017,A Location in patent:Paragraph 0136; 0154-0156
![Dimethyl [2-oxo-3-[3-(trifluoromethyl)phenoxy]propyl]phosphonate](/CAS/GIF/54094-19-8.gif)
54094-19-8
131 suppliers
$65.00/1g
![[1,1'-Biphenyl]-4-carboxylic acid, (3aR,4R,5R,6aS)-hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2-oxo-2H-cyclopenta[b]furan-5-yl ester](/CAS/20180702/GIF/114488-91-4.gif)
114488-91-4
16 suppliers
inquiry
![[1,1'-Biphenyl]-4-carboxylic acid (3aR,4R,5R,6aS)-4-formylhexahydro-2-oxo-2H-cyclopenta[b]furan-5-yl ester](/CAS2/GIF/38754-71-1.gif)
38754-71-1
43 suppliers
inquiry
![[1,1'-Biphenyl]-4-carboxylic acid, (3aR,4R,5R,6aS)-hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2-oxo-2H-cyclopenta[b]furan-5-yl ester](/CAS/20180702/GIF/114488-91-4.gif)
114488-91-4
16 suppliers
inquiry
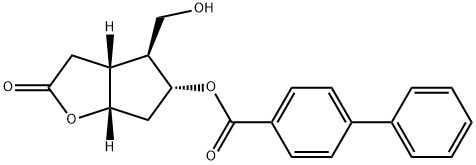
31752-99-5
246 suppliers
$19.00/250mg
![[1,1'-Biphenyl]-4-carboxylic acid, (3aR,4R,5R,6aS)-hexahydro-4-[(1E,3R)-3-hydroxy-4-[3-(trifluoromethyl)phenoxy]-1-buten-1-yl]-2-oxo-2H-cyclopenta[b]furan-5-yl ester](/CAS/20180702/GIF/114488-91-4.gif)
114488-91-4
16 suppliers
inquiry