
(S)-3-(2-Chloro-pyrimidin-4-ylamino)-pyrrolidine-1-carboxylic acid tert-butyl ester synthesis
- Product Name:(S)-3-(2-Chloro-pyrimidin-4-ylamino)-pyrrolidine-1-carboxylic acid tert-butyl ester
- CAS Number:1146159-96-7
- Molecular formula:C13H19ClN4O2
- Molecular Weight:298.7686
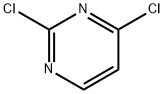
3934-20-1
714 suppliers
$9.00/1g
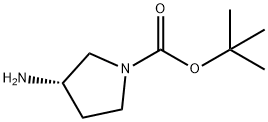
147081-44-5
330 suppliers
$14.00/1g

1146159-96-7
4 suppliers
inquiry
Yield:1146159-96-7 90%
Reaction Conditions:
with triethylamine in N,N-dimethyl acetamide at 0 - 20; for 24 h;
Steps:
21-A
Synthesis 21 -A; (S)-tert-Butyl 3-(2-chloropyrimidin-4-ylamino)pyrrolidine-1-carboxylate; (S)-(-)-1-Boc-3-aminopyrrolidine (0.375 g, 2.014 mmol) and triethylamine (0.561 mL,4.028 mmol) were added to a solution of 2,4-dichloropyrimidine (0.300 g, 2.014 mmol) in DMA (12 mL) at 0°C. After 24 hours at room temperature, water (150 mL) was added and the aqueous phase was extracted with ethyl acetate (3 x 40 mL). The combined organic extracts were dried (MgSO4) and concentrated. The crude product was purified by silica gel chromatography (5% methanol in dichloromethane) to give the title compound as an oil (0.540 g, 90%).LC-MS (LCT2) m/z 243 [M+H-'Bu*], R, 4.54 minutes. 1H NMR (CDCI3) δ 7.99 (br s, 1H), 6.31 (d, J 6.0 Hz, 1 H)1 5.99 (br s, 1 H), 4.55 (br s, 1 H), 3.68 (dd, J 11.5, 6.0 Hz, 1 H), 3.49- 3.42 (m, 2H)1 3.33-3.21 (m, 1 H), 2.26-2.18 (m, 1 H)1 1.99-1.88 (m, 1 H), 1.45 (s, 9H).
References:
WO2009/53694,2009,A1 Location in patent:Page/Page column 121