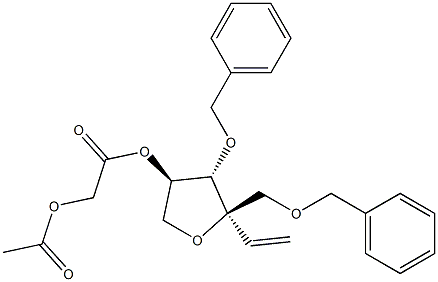
(3R,4S,5R)-2-(acetyloxy)-4-(benzyloxy)-5-[(benzyloxy)methyl]-5-ethenyloxolan-3-yl acetate synthesis
- Product Name:(3R,4S,5R)-2-(acetyloxy)-4-(benzyloxy)-5-[(benzyloxy)methyl]-5-ethenyloxolan-3-yl acetate
- CAS Number:1146197-36-5
- Molecular formula:C25H28O7
- Molecular Weight:440.49
Yield:1146197-36-5 58%
Reaction Conditions:
with sulfuric acid;acetic acid at 20; for 3 h;
Steps:
Synthesis of compound (3R,4S,5R)-4-(benzyloxy)-5-(benzyloxymethyl)-5-vinyl-tetra hydrofuran-2,3-diyl diacetate (3)
To a solution of 2 (0.72 g, 1.82 mmol) in AcOH/Ac2O (16.5 mL/2 mL) was added H2SO4 (0.35 mL).
After stirring at room temperature for 3 h, the reaction mixture was poured into ice-water and then treated with saturated aqueous NaHCO3 (5 mL).
The organic phase was extracted with ethyl acetate was washed with water, brine solution, dried (Na2SO4) and evaporated to dryness under reduced pressure.
Chromatography column (Chromatography (petroleum ether:ethyl acetate 20:1 to 10:1) to afford the product 3 (0.471 g, 58%) as a white solid. j0198] EC-MS: (M+Na)=463.1j0199] ‘H NMR (300 MHz, CDC13) ? 7.32-7.30 (m, 1OH), 6.191 (s, 1H), 6.00-5.94 (m, 1H), 5.50 (dd, J=1.5 and 17.1 Hz, 1H), 5.31-5.23 (m, 2H), 4.66 (d, J=11.7 Hz, 1H), 4.51-4.42 (m, 4H), 3.42 (s, 2H), 2.06 (s, 3H), 1.87 (s, 3H).
References:
US2016/264611,2016,A1 Location in patent:Paragraph 0196; 0197; 0198; 0199
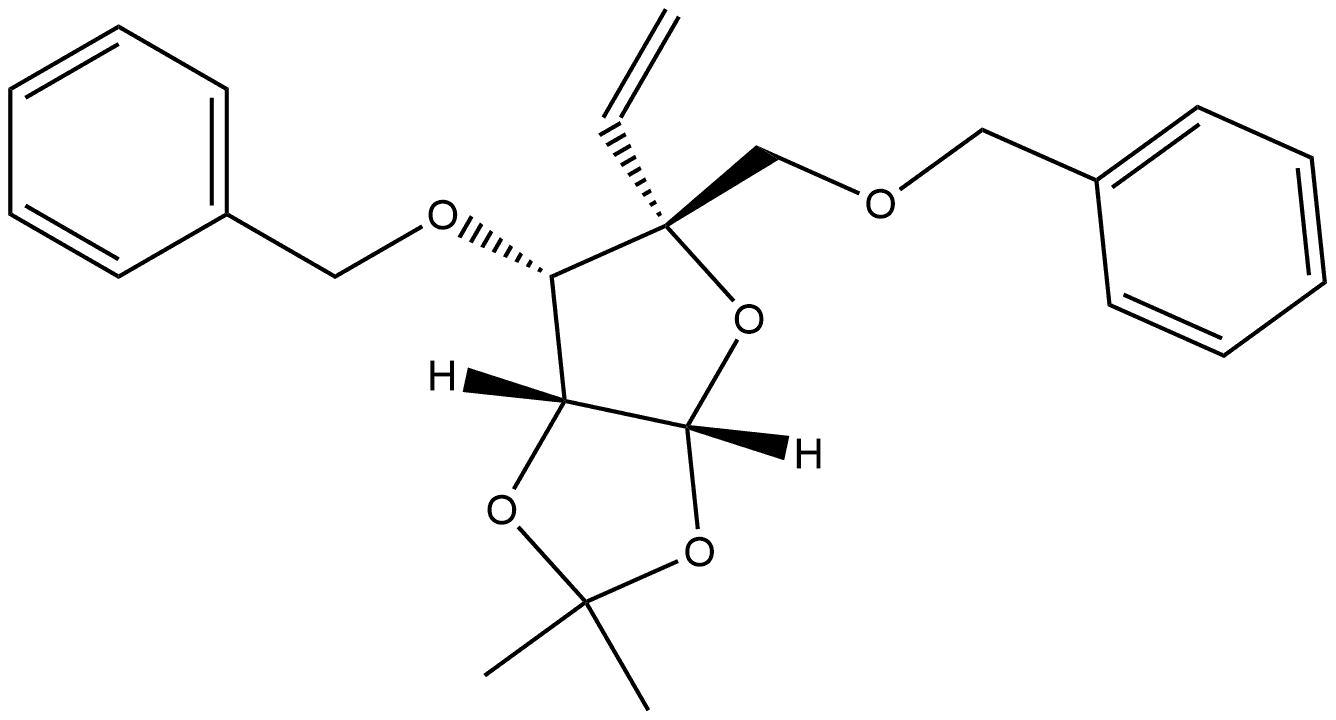
![L-arabino-Pentodialdo-5,2-furanose, 4,5-O-(1-methylethylidene)-2-C-[(phenylmethoxy)methyl]-3-O-(phenylmethyl)-, (5R)-](/CAS/20200611/GIF/233266-76-7.gif)
233266-76-7
7 suppliers
inquiry
![(3R,4S,5R)-2-(acetyloxy)-4-(benzyloxy)-5-[(benzyloxy)methyl]-5-ethenyloxolan-3-yl acetate](/CAS/20180527/GIF/1146197-36-5.gif)
1146197-36-5
14 suppliers
inquiry